Figure 8. ATP8A2 contains phosphatidylserine translocase activity.
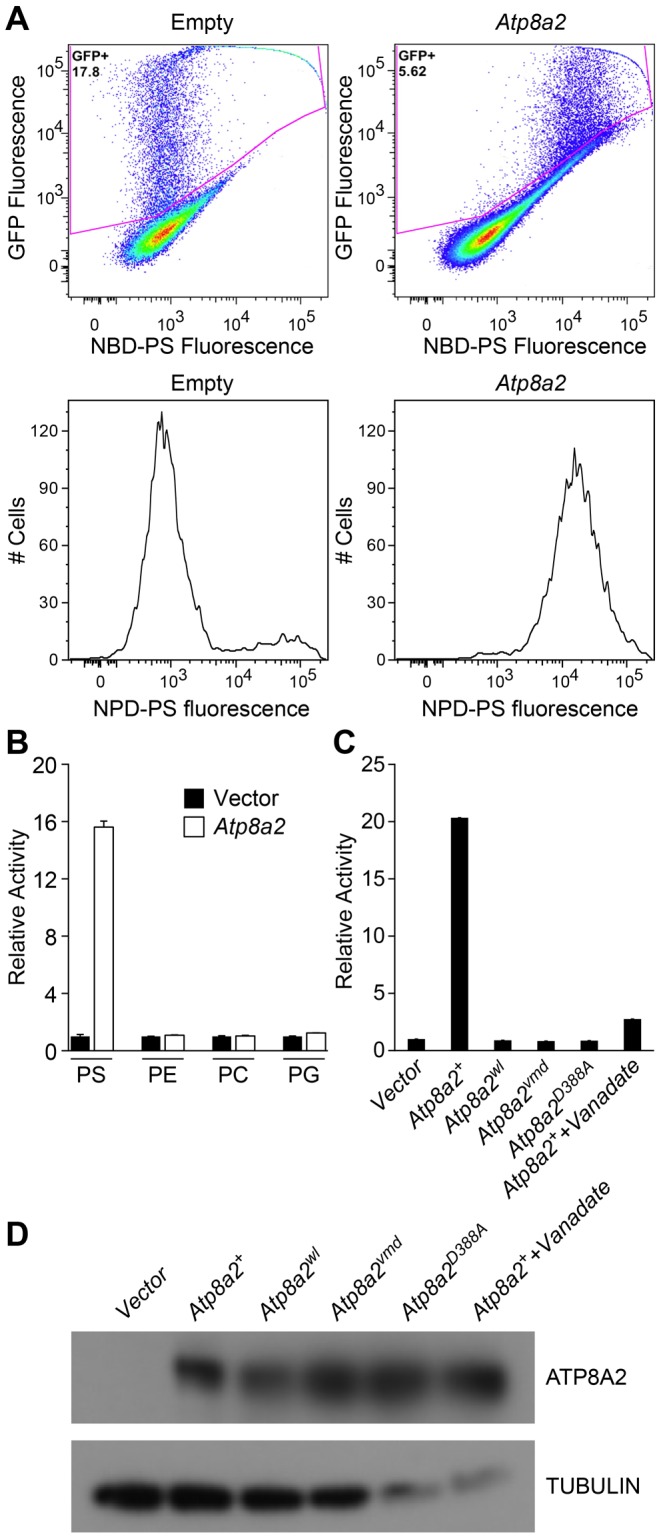
(A) Internalization of NBD phospholipids by UPS-1 cells transfected with a control vector (Empty) or plasmid expressing Atp8a2. Expression of ATP8A2 leads to a population of UPS-1 cells with increased NBD-PS uptake (Top right panel). Representative numbers of NBD-PS-labeled UPS-1 cells are shown (Bottom panel). The X-axis represents NBD-PS fluorescence intensity of GFP-positive cells; cells transfected with pcDNA62-Atp8a2 vector shows increased fluorescence intensity (bottom right panel), compared to cells transfected with empty vector (bottom left panel). (B) ATP8A2 specifically translocates NBD-PS across the plasma membrane of UPS-1 cells. Lipid translocation activity is shown as a percentage of NBD-lipid fluorescence intensity relative to control empty vector (defined as 1). Results are representative data from three independent experiments. No translocation activity was observed for NBD-phospholipids PE- phosphatidyletholamine, PC- phosphatidylcholine, or PG- phosphatidylglycerol. Four independent samples were assessed for each group. (C) Mutant proteins encoded by wl and vmd mutant mice are non-functional for PD translocation. Lipid translocation activity of ATP8A2wl and ATP8A2vmd encoded by wl and vmd mutant animals are similar to the vector control. A single D→A point mutation (ATtp8a2D388A) in the conserved DKLTG motif completely abolishes the lipid translocation activity of ATP8A2. A chemical ATPase inhibitor sodium vanadate significantly reduces ATP8A2 activity. (D) ATP8A2 protein levels were similar in all transfected cells (other than those transfected with empty vector) as assessed by western blotting analysis using the ATP8A2 antibody.
