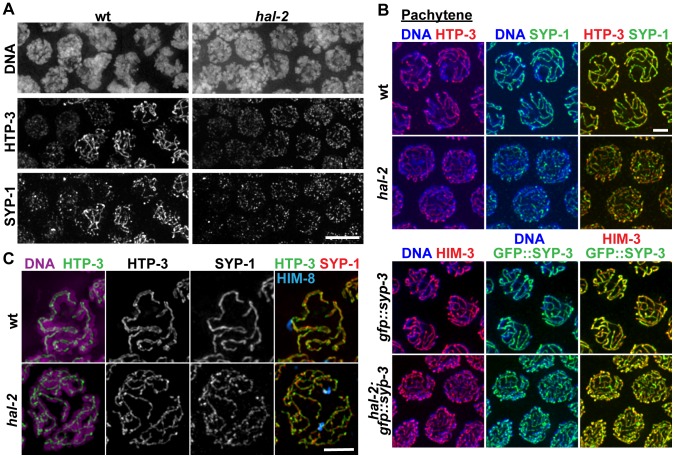
Figure 3. SYP proteins are loaded onto the axes of unpaired homologs in hal-2 mutants.
(A) Portions of wild-type and hal-2 germ lines extending from premeiotic nuclei (left) to nuclei progressing into meiosis (right), stained with antibodies against HTP-3 (LE component) and SYP-1 (CR protein). In both germ lines, most nuclei in which HTP-3 is detected as discrete axial structures show SYP-1 colocalizing with most HTP-3 stretches, indicating that CR components are competent to load onto the axes of unpaired homologs soon after meiotic entry in the hal-2 mutant. Bar, 5 µm. (B) Co-immunolocalization of LE components (HTP-3 [top] or HIM-3 [bottom]) and CR proteins (SYP-1 [top] or GFP::SYP-3 [bottom]) in wild-type and hal-2 nuclei at mid/late pachytene. LE components and SYP proteins colocalize at the interface between DAPI-stained aligned homologs in wild-type nuclei, whereas SYP proteins colocalize with LE components along the lengths of unpaired chromosomes in hal-2 mutant nuclei. Bar, 2 µm. (C) Localization of LE component HTP-3, CR protein SYP-1 and DAPI-stained chromatin visualized using 3D-SIM; for both genotypes, pairing status of the X chromosomes is indicated by HIM-8 immunostaining (imaged without 3D-SIM). In the wild-type nucleus, two resolvable parallel tracks of HTP-3 flanking a single track of SYP-1 are detected along much of the lengths of the aligned homolog pairs, reflecting the presence of SCs linking pairs of LEs that are separated by a distance of approximately 100 nm [29], [49]. In the hal-2 nucleus, both SYP-1 and HTP-3 are detected as single tracks at the sister chromatid interface of individual unpaired chromosomes. Bar, 2 µm.