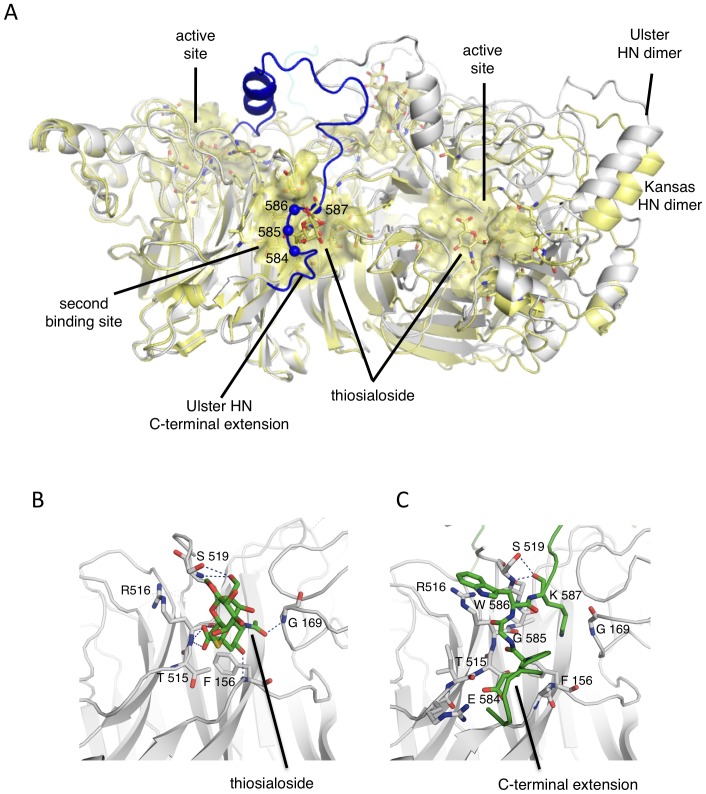
Figure 8. Residues 584–587 of the C-terminal extension engage the second sialic acid binding site at the NA domain dimer interface.
(A) Superposition of Ulster (grey) and Kansas (yellow) NA domain dimers, highlighting the location of the second sialic acid binding site at the dimer interface. The Kansas and Ulster dimers superimpose with a RMSD of 1.6 Å. The active sites and second binding site are indicated with transparent yellow surfaces and labeled. Residues 584–587 of the C-terminal extension cover the second receptor binding site and are highlighted with blue Cα spheres. Comparisons of the detailed interactions of thiosialoside (B) and the C-terminal extension residues (C) at the second binding site.