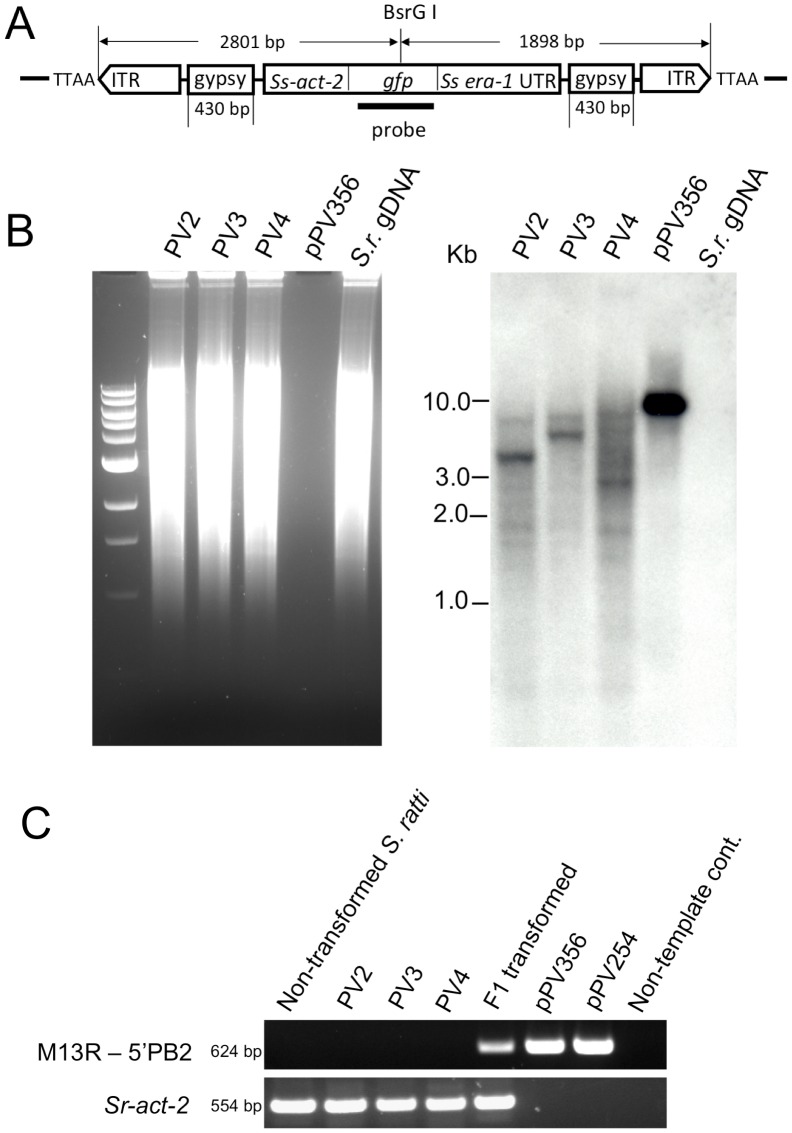
Figure 4. Transgene specific sequences are widely distributed in restriction digests of genomic DNA from three stable lines of transgenic S. ratti.
Southern hybridization of genomic DNA (gDNA) of S. ratti from stably transformed lines probed for the gfp coding sequence. A) Transgene diagram showing position of the probe used for Southern hybridization analysis and the single restriction site for BsrGI, the enzyme used for restriction digestion of gDNA. B) Southern blot of BsrGI digests of gDNA from pooled free-living adults from three independent integrated lines (PV2, PV3 and PV4). The positive control lane is a blot of a BsrGI digest of donor plasmid pPV356, and the negative control is a blot of a BsrGI digest of gDNA from non-transformed S. ratti free-living adults (S.r. gDNA). Note gfp hybridization signals in multiple restriction fragments in gDNA from each of the three integrated lines. A single hybridizing band of 8.1 kb appears as predicted in the positive control digest of pPV356. No gfp-specific signal is detected in the negative control digest of gDNA from non-transformed S. ratti. C) Gel analysis of PCR products from genomic DNA templates from non-transformed control parasites, parasites from each of three stable lines, PV2, PV3 and PV4, with integrated, stably expressed transgenes and F1 progeny of free-living female worms microinjected with donor plasmid pPV356 and helper plasmid pPV402. Also included are PCR products from control reactions with plasmids pPV356 and pPV254 as templates and from a reaction from which template was omitted. Upper gel image depicts 624 bp amplification products resulting from a forward primer hybridizing to the M13 reverse priming site in the vector and a reverse primer hybridizing within the transposon sequence. Lower gel image is a loading control showing the expected 554 bp amplification product from reactions with primers specific for the constitutively expressed cellular actin-encoding gene Sr-act-2.