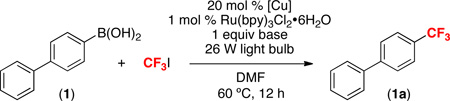
Table 1.
Optimization of Reaction Between 1 and CF3I[a]
 | |||
|---|---|---|---|
| Entry | [Cu] | Base | Yield |
| 1 | Cu(OTf)2 | K2CO3 | 14% |
| 2 | [Cu(OTf)]2•C6H6 | K2CO3 | 28% |
| 3 | Cul | K2CO3 | 34% |
| 4 | Cu | K2CO3 | 40% |
| 5 | Cu(OAc)2 | K2CO3 | 68% |
| 6 | CuOAC | K2CO3 | 76% |
| 7 | CuOAC | NaOAc | 34% |
| 8 | CuOAC | KF | 50% |
| 9 | CuOAC | none | 6% |
| 10[b] | CuOAC | K2CO3 | 1% |
| 11[c] | none | K2CO3 | 3% |
| 12[d] | CuOAC | K2CO3 | 3% |
General conditions: substrate (0.05 mmol, 1 equiv), CF3I (5 equiv), [Cu] (0.2 equiv), Ru(bpy)3Cl2•6H2O (0.01 equiv), base (1 equiv), DMF (0.17 M in substrate), 60 °C, 12 h, 26 W compact fluorescent light bulb. 19F NMR yield.
General conditions, but with no light.
General conditions, but with no CuOAc.
General conditions, but with no Ru(bpy)3Cl2•6H2O.
