Table 2.
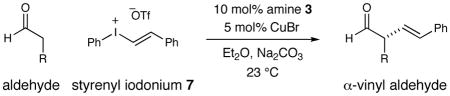
| |||
|---|---|---|---|
| 1 |
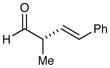 82% yield 94% ee 82% yield 94% ee |
2d,e |
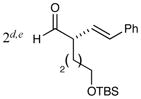 83% yield 96% ee 83% yield 96% ee |
| 3 |
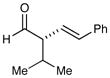 75% yield 99% ee 75% yield 99% ee |
4 |
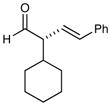 79% yield 99% ee 79% yield 99% ee |
| 5 |
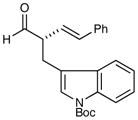 81% yield 91% ee 81% yield 91% ee |
6 |
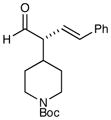 73% yield 96% ee 73% yield 96% ee |
| 7 |
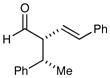 84% yield ≥20:1 dr 84% yield ≥20:1 dr |
8f |
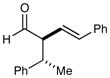 81% yield ≥20:1 dr 81% yield ≥20:1 dr |
Absolute configuration assigned by chemical correlation or analogy.
Isolated yield of the corresponding alcohol.
Enantiomeric excesss determined by chiral HPLC analysis of the corresponding alcohol.
Using 20 mol% 3.
Using 20 mol% CuBr.
Using (S,S)-3 catalyst.
