Table 2.
Stereoconvergent Negishi Phenylations of Racemic α-Bromonitrilesa
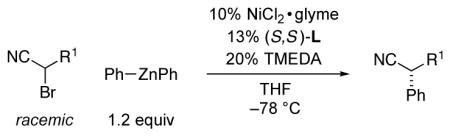
| |||
|---|---|---|---|
| entry | R1 | ee (%) | yield (%) b |
| 1 | i-Pr | 92 | 77 |
| 2 |
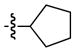
|
92 | 98 |
| 3 |
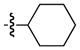
|
92 | 92 |
| 4c |
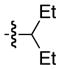
|
92 | 92 |
| 5 |
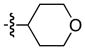
|
92 | 94 |
| 6 |
|
90 | 96 |
| 7 |
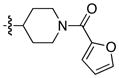
|
85 | 95 |
| 8 |
|
91 | 94 |
| 9 |
|
90 | 94 |
| 10 | Me | 82 | 67 (83)d |
| 11 |
|
78 | 88 |
| 12 |
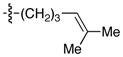
|
76 | 94 |
All data are the average of two experiments.
Yield of purified product.
Reaction temperature: −60 °C.
Yield determined by GC analysis versus a calibrated internal standard.
