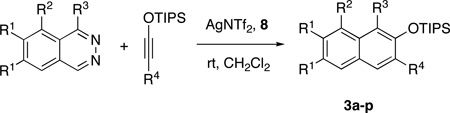
Table 2.
Scope of AgNTf2-Catalyzed Cycloaddition Reactionsa
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Catalyst mol% |
Time (h) |
Product | Yieldb (%) |
Entry | Catalyst mol% |
Time (h) |
Product | Yieldb (%) |
| 1 | 1.0 | 2 | 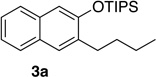 |
82 | 9 | 1.0 | 1 | 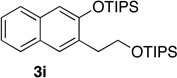 |
81 |
| 2 | 2.0 | 1.5 | 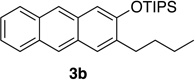 |
72 | 10d | 2.0 | 3 | 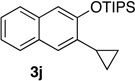 |
75 |
| 3 | 1.0 | 7 | 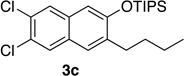 |
78 | 11e | 2.0 | 3 | 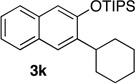 |
83 |
| 4 | 2.0 | 3 | 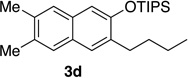 |
84 | 12d | 10 | 22 | 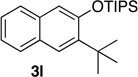 |
67 |
| 13d,f | 10 | 7 | 80 | ||||||
| 5c | 2.0 | 4 | 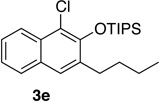 |
67 | 14 | 1.0 | 1 | 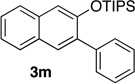 |
95 |
| 15 | 0.5 | 3 | 92 | ||||||
| 6c | 2.0 | 4 | 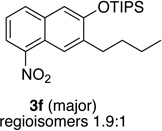 |
74 | 16 | 1.0 | 3 | 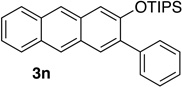 |
84 |
| 7d | 2.0 | 3 | 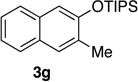 |
73 | 17 | 1.0 | 2 | 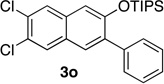 |
85 |
| 8e | 2.0 | 1 | 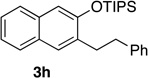 |
70 | 18 | 1.0 | 6 |  |
94 |
Reactions were carried out using 0.5 or 1.0 mmol of diazine and 1.3 equiv of the siloxy alkyne with 8AgNTf2 ratio of 1.1:1.
Isolated yields.
For the determination of the regiochemistry of the product, see the Supporting Information.
2.0 equiv of siloxy alkyne was used.
1.5 equiv of siloxy alkyne was used.
The reaction was carried out in refluxing CH2Cl2.
