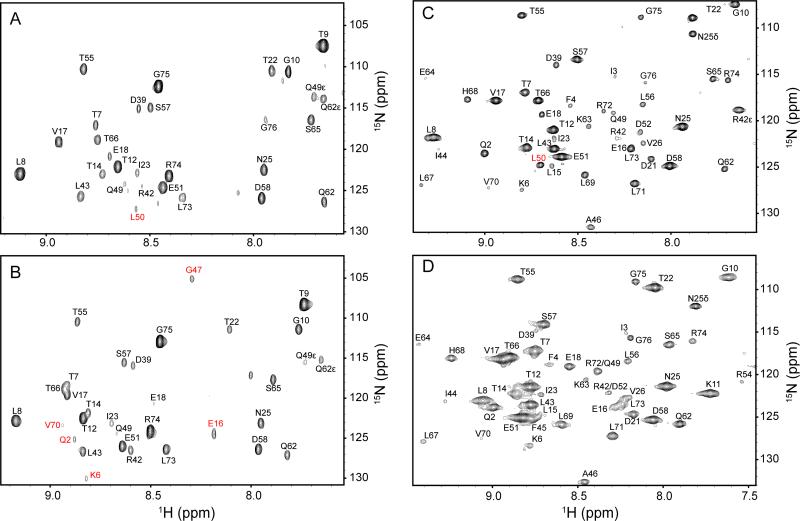
Figure 6.
The absence of water in the interior of the protein at high hydrostatic pressure. Indirect 1H planes at the water resonance of three-dimensional 15N-resolved NOESY spectra of 15N, 2H-labeled ubiquitin in aqueous (4.7 ppm) and encapsulated in AOT reverse micelles in liquid pentane (4.5 ppm). Panels A and B are ubiquitin in free aqueous solution at 1 bar and 2.5 kbar, respectively. Panels C. and D are encapsulated ubiquitin in pentane at at 1 bar and 2.5 kbar, respectively. Differences in cross peak position result from pressure dependent changes in amide (1H,15N) chemical shifts, while differences in linewidth arise from the increase in solvent viscosity with increasing pressure. Amide hydrogen-water cross peaks of L50 that disappear at high pressure both in aqueous and in encapsulated conditions are indicated in Panels A and C in red. The cross peaks of Q2, K6, E16, G47 and V70 that appear at high pressure only in free aqueous solution are indicated in B in red. There is no evidence for penetration of water into the protein at ambient or elevated pressure. Only amide hydrogens within NOE-detection distance of the surface of the protein show NOEs to the water resonance at either pressure.