Figure 3.
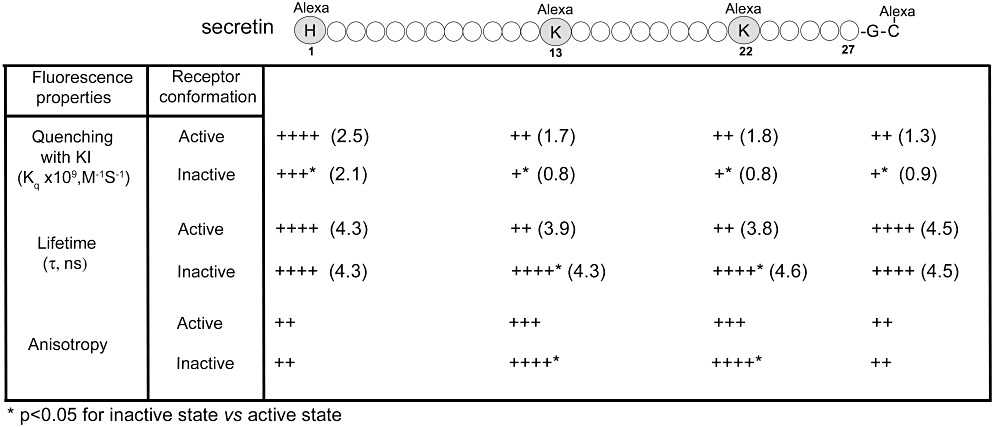
Fluorescence analysis using secretin probes bound to the secretin receptor. Shown are the positions of incorporation of fluorescent alexa488 into the amino terminus of secretin (adjacent to position 1), the carboxyl terminus of secretin (as a two-residue extension beyond position 27), and in the mid-region of secretin in positions 13 and 22. These probes were utilized in studies of the ability to quench the fluorescence with hydrophilic KI, the fluorescence lifetimes and the fluorescence anisotropy, as indications of the characteristics of the microdomains occupied by each fluorophore in the probes while bound to the secretin receptor (Harikumar et al., 2006a). The G protein-uncoupled low affinity state was achieved by incubation with GppNHp, and significant differences in each of the fluorescence characteristics relative to those when the receptor was in the high affinity state are noted. Fluorescence at the amino terminus of the peptide ligand was more readily quenched than that at any other position. The fluorescence of each of the probes was more readily quenched when the receptor was in its G protein-coupled high affinity state than in its low affinity state. For both lifetimes and anisotropy, only the mid-region probes reflected differences relative to receptor conformation, with the probe fluorescence while the receptor was in its high affinity state exhibiting shorter lifetimes and lower anisotropy than when the receptor was in its low affinity state.
