Abstract
BACKGROUND AND PURPOSE
The neuromodulatory effects of the gut-neuropeptide neurotensin on sympathetic vasomotor tone, central respiratory drive and adaptive reflexes in the spinal cord, are not known.
EXPERIMENTAL APPROACH
Neurotensin (0.5 µM–3 mM) was administered into the intrathecal (i.t.) space at the sixth thoracic spinal cord segment in urethane-anaesthetized, paralysed, vagotomized male Sprague–Dawley rats. Pulsatile arterial pressure, splanchnic sympathetic nerve activity (sSNA), phrenic nerve activity, ECG and end-tidal CO2 were recorded.
KEY RESULTS
Neurotensin caused a dose-related hypotension, sympathoinhibition and bradycardia. The maximum effects were observed at 3000 µM, where the decreases in mean arterial pressure (MAP), heart rate (HR) and sSNA reached −25 mmHg, −26 beats min−1 and −26% from baseline, respectively. The sympathetic baroreflex was enhanced. Changes in central respiratory drive were characterized by a fall in the amplitude of the phrenic nerve activity. Finally, administration of SR 142948A (5 mM), a potent, selective antagonist at neurotensin receptors, caused a potent hypotension (−35 mmHg), bradycardia (−54 beats min−1) and sympathoinhibition (−44%). A reduction in the amplitude and frequency of the phrenic nerve activity was also observed.
CONCLUSIONS AND IMPLICATIONS
The data demonstrate that neurotensin plays an important role in the regulation of spinal cardiovascular function, affecting both tone and adaptive reflexes.
Keywords: neurotensin, intrathecal, blood pressure, sympathetic nerve activity, baroreflex, SR 142948A, phrenic nerve activity
Introduction
The sympathetic nervous system and kidneys are vital for the tonic and reflex maintenance of arterial blood pressure and to enable appropriate circulation of blood to different tissues (Guyton, 1991; Guyenet, 2006). Disorders of either the sympathetic nervous system, or kidneys, may cause hypertension (DiBona, 2002; Chobanian et al., 2003; Hall, 2003; Katholi and Rocha-Singh, 2009; Schlaich et al., 2004). In central cardiorespiratory regulation, several key brainstem nuclei including the nucleus of the solitary tract and the rostral ventrolateral medulla are critical for the integration of adaptive autonomic reflexes, such as baro- and chemoreflexes (Pilowsky and Goodchild, 2002).
Neurotensin is a 13-amino-acid peptide originally isolated from bovine hypothalamus (Carraway and Leeman, 1973). Both the digestive system and the CNS synthesize neurotensin (Carraway et al., 1982; Sarrieau et al., 1985; Kislauskis et al., 1988). In vivo and in vitro activations of two neurotensin GPCRs (NTS1 and NTS2) and a neurotensin non-GPCR (i.e. NTS3) result in analgesia, inhibition of gastric acid secretion, modulation of digestive tract peristalsis and stimulation of growth in a variety of normal and cancer cells (Osumi et al., 1978; Goedert et al., 1984; Mule et al., 1996; Dubuc et al., 1999; Vincent et al., 1999; Tyler-McMahon et al., 2000; Stolakis et al., 2010). In the CNS, neurotensin interacts with the dopaminergic, cholinergic, 5-hydroxytryptaminergic and noradrenergic systems (Binder et al., 2001; Nieoullon, 2002; St-Gelais et al., 2006).
Although neurotensin and its receptors are localized to nuclei in the spinal cord (Stanzione and Zieglgansberger, 1983; Faull et al., 1989; Sarret et al., 2005; Roussy et al., 2009), little is known about the role of this peptide in modulating sympathetic function. To investigate the role of neurotensin in cardiorespiratory function in the spinal cord, we administered neurotensin intrathecally (i.t.) in anaesthetized rats. We aimed to examine the effect of neurotensin on resting sympathetic tone, mean arterial blood pressure and other cardiovascular parameters. We also tested whether or not neurotensin affects adaptive autonomic reflexes, the baroreflex and central chemoreflex, since earlier studies suggest that neurotensin can either suppress (Chen et al., 1990) or enhance (Kubo and Kihara, 1990) barosensitivity in anaesthetized rats. Effects on central inspiratory activity were determined by recording phrenic nerve discharge. Finally, we used SR 149248A, to block the actions of neurotensin at neurotensin 1 (NTS1) and 2 (NTS2) receptors (Schaeffer et al., 1998), to determine if a tonic neurotensin input is present. The main findings of this study are that i.t. neurotensin causes a dose-related decrease in mean arterial pressure (MAP), heart rate (HR) and splanchnic sympathetic nerve activity (sSNA). The amplitude of phrenic nerve discharge (PNamp) was also decreased by neurotensin. Adaptive reflexes were differentially affected: barosensitivity was enhanced, while MAP responses to hypercarbia were attenuated. SR 142948A, an antagonist at NTS1 and NTS2 caused neurotensin-like effects that manifest in hypotension, bradycardia, sympathoinhibition and a decrease in central inspiratory drive. The data show that neurotensin plays an important role in the modulation of cardiorespiratory functions and autonomic adaptive reflexes at the level of the spinal cord.
Methods
All animal care and experimental procedures were approved by the Macquarie University Animal Ethics Committee and carried out under the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/ea16.pdf).
General preparation
Male Sprague–Dawley rats (n= 23; 400–600 g) were anaesthetized with urethane (1.3 g·kg−1, 10% saline solution, i.p.). Atropine (50 µg) was given initially to reduce bronchial and gastric secretions. Sodium chloride anhydride, glucose, urethane, sodium nitroprusside, phenylephrine hydrochloride, potassium chloride anhydride and neurotensin were purchased from Sigma-Aldrich (Sydney, Australia). SR 1492984A was purchased from Axon Medchem (Groningen, The Netherlands). Pancuronium and atropine were purchased from Astra (Sydney, Australia). Changes in blood pressure (>10 mmHg) or HR (>15 beats min−1) to painful stimuli (paw pinch), or presence of corneal reflex, were assessed approximately every 15 min to test depth of anaesthesia. Supplemental urethane (30 mg in 10% solution i.v.) was given when necessary. Temperature was monitored via rectal probe and was regulated via a homeothermic blanket to values between 36.5 and 37.5°C. The right femoral artery was cannulated for blood pressure recording and blood gas analysis (Idexx, Sydney, Australia), while the left jugular external vein was cannulated for i.v. drug administration (PVC tubing: OD, 0.96 mm, ID, 0.58 mm; Critchley Electrical Products, Castle Hill, Australia). The trachea was exposed, and a catheter (from a 14G i.v. catheter) was inserted through an incision below the larynx. During tracheal exposure, the right carotid sheath was identified, and the right vagus nerve was isolated and cut; 5% glucose in saline was administered i.v. for maintenance of normovolaemia. The left phrenic and greater splanchnic nerves were approached from a dorsolateral incision and cut distally. A silk tie (5/0; Pearalls Ltd., Taunton, UK) was placed on the distal end of each nerve. The nerves were preserved with soaked cotton wool. At the start of the experiment, nerves were placed on electrodes and maintained and electrically isolated, under paraffin oil. Nerve activity was recorded with bipolar silver electrodes. Nerve activity was filtered (100–3000 Hz) and amplified (500−2000 Hz; CWE, BMA, Ardmore, OK). Data were recorded using a CED Micro1401Mark II. The ECG was monitored, and the signal was filtered (100–3000 Hz) and amplified (500−2000 Hz; CED Micro1401Mark II). Rats were then positioned in a stereotaxic frame. The muscles attached to the superior nuchal line were detached, and the atlanto-occipital membrane was exposed. The subarachnoid space of the thoracic spinal cord was identified via an incision on the atlanto-occipital membrane, the dura and arachnoid mater. An i.t. catheter (PVC tubing: OD, 0.5 mm, ID, 0.2 mm; Critchley Electrical Products) was then inserted from the cisterna magna caudally, so that the tip lay at T6. Backflow of cerebrospinal fluid following incision of the atlanto-occipital membrane and into the catheter following insertion confirmed accurate placement in the subarachnoid space. The left phrenic and left vagus nerves were cut immediately before placement of the phrenic nerve on the electrodes. Following completion of surgery, neuromuscular blockade and artificial ventilation were commenced (pancuronium i.v.; 0.4 mg initially, then 0.3 mg·h−1). Ventilation was commenced at between 3.5 and 4 L min−1, with a frequency of 60 and 75 cycles min−1, depending on the weight of the rat (Capstar-100; CWE Inc., Ardmore, PA, USA). Depth and frequency of ventilation were adjusted in order to maintain end-tidal CO2 between 3.5% and 4.5%, arterial blood gases were assessed immediately after commencing ventilation, and at least two to three times subsequently, during the experiment; changes in ventilation were made as needed in order to achieve normal values. Manipulations were ceased during data recordings to evaluate drug effects on breathing parameters. At the end of the experiment, rats were killed with i.v. potassium chloride (3 M, 0.5 mL). Verification of i.t. catheter tip position was achieved postmortem via application of India ink and thoracic laminectomy.
Drugs
Neurotensin (NH2-pGlu-Leu-Tyr-Glu-Asn-Lys-Pro-Arg-Arg-Pro-Tyr-Ile-Leu-COOH; range: 0.5, 1, 10, 75, 250, 750 and 3000 µM) and urethane (ethyl-carbamate) were dissolved in saline. SR 142948A (5 mM) was dissolved in dimethyl sulphoxide (∼1%) and then diluted in saline to reach final concentrations. Dimethyl sulphoxide (∼1%) in saline was used as vehicle for SR 149248A. Vehicle (normal saline 15 µL), neurotensin (10 µL flushed with 5 µL control) and SR 1492984A (10 µL flushed with 5 µL saline; Sigma-Aldrich) were delivered through a glass syringe (25 µL; Hamilton, Reno, NV, USA). Sodium nitroprusside (5 µg) and phenylephrine hydrochloride (5 µg) were used to test the baroreflex. A hypercarbic gas mix (10% CO2 in 90%O2, administered for 90 s; BOC, Sydney, Australia) was used to test the central chemoreflex.
Experimental protocol
Rats were divided into three groups. In the first group (n= 8), vehicle (15 µL) and neurotensin (increasing concentrations starting from 0.5 µM) were tested. At the completion of surgery, baseline data were recorded for at least 30 min. Vehicle was then injected, and data were recorded for a further 60 min. This was followed by administration of the lowest neurotensin dose (0.5 µM). Data were then recorded for another 60 min. At the end of this period, the same procedure was repeated six times, except that a higher neurotensin dose (1–3000 µM) was administered each time.
In the second group of animals (n= 8), baseline data were again recorded for at least 30 min. This was followed by i.t. application of vehicle. Data were recorded for at least 1 h. At the end of this period, 75 µM neurotensin was administered i.t., and data were recorded for a further 60 min. Finally, 3000 µM neurotensin was administered i.t., and data were recorded for at least an hour. Around 20 min following the administration of vehicle or neurotensin (75, 3000 µM), the baroreflex was examined. Finally, the chemoreflex responses were examined at 30 min and then 40 min after the i.t. application of vehicle and neurotensin (75, 3000 µM).
In the third group of rats (n= 7), vehicle, SR 142948A (5 mM) and SR 142948A (5 mM) + 75 µM neurotensin were tested. Animals were given a period of at least 30 min to recover from surgery, during which baseline data were recorded. At the end of the recovery period, vehicle was applied i.t., and data were recorded for 60 min. This was followed by i.t. administration of SR 1492984A. Data were then recorded for another 60 min. SR 1492984A was then re-administered followed by neurotensin i.t. application within 5–10 min. Data were recorded for at least an hour.
Data analysis
Data were analysed off-line with Spike2 software (v. 7.02a, CED, Cambridge, England). Pulsatile blood pressure was integrated (τ= 10 s) to derive MAP. sSNA was rectified and integrated (τ= 1 s). Integrated sSNA was calibrated as percentage activity, where baseline activity at the start of recordings was designated as 100%, and the lowest stable baseline approximately 5 min after killing was designated as 0%. Central respiratory drive was measured from PNamp and frequency (PNf) of phrenic nerve activity. Phrenic nerve activity was rectified, smoothed (τ= 0.05 s), calibrated as an area under the curve and normalized as a percentage. PNf was acquired as the number of phrenic bursts, while PNamp represented the peak values of each burst of phrenic nerve discharge. Peak end-tidal CO2 (etCO2) was recorded. ECG parameters (PR interval, QRS complex and ST interval) were derived from ECG recordings and averaged over 5 min intervals. HR was derived from the ECG. Cardiac contractility was estimated as the first derivative of blood pressure (dP/dT) (Ifuku et al., 1994). Maximum responses were expressed as absolute changes in MAP, HR and PNf, and % changes in sSNA, PNamp, and etCO2 from baseline values. Data were summarized, and statistical analyses were conducted using Excel (Microsoft, Redmont, WA, USA) and (GraphPad Prism, v. 5.03, La Jolla, CA, USA). In the first group of animals, cumulative data for MAP were plotted as dose versus time in 1 min intervals every 5 min. Two-way anova was used for statistical comparison. The IC50 for neurotensin was derived from a log-dose–response curve. In the second and third groups, maximal effects following drug administration were measured, averaged and compared with a one-way anova followed by Student's t-test with Bonferroni's correction. For baroreflex analysis, pulsatile arterial pressure and integrated sSNA were plotted to a sigmoid curve, calculated from the Boltzmann equation: Y= Lower +[(Upper − Lower)/(1 + 10(MidPoint-X) − Gain Coefficient)]. In the equation, ‘Lower’ is the value of the ordinate axis at the lower plateau, ‘Upper’ is the value of the ordinate axis at the upper plateau, ‘MidPoint’ is the value on the abscissa axis midway between ‘upper’ and ‘lower’ and ‘Gain Coefficient’ represents the slope of the curve. Responses in the top, bottom plateau, minimum gain, the range of sSNA, the range of blood pressure, threshold and saturation were analysed. Baroreflex and chemoreflex data were analysed with a one-way anova followed by Student's t-tests with Bonferroni's correction. All values are mean ± SEM. P < 0.05 was considered statistically significant.
Results
I.t. neurotensin causes a dose-dependent hypotension
I.t. neurotensin caused a dose-dependent decrease in mean arterial blood pressure. The threshold dose at which hypotension was first observed was 75 µM (Figure 1). Hypotension developed gradually over the first 5 min following i.t. injection of neurotensin. The maximum level of hypotension (−30 ± 3 mmHg) was reached within 15–20 min following neurotensin administration (Figure 1B, P < 0.01). Hypotension then persisted for at least 60 min. Subsequent, higher doses of neurotensin (250, 750 and 3000 µM) caused further increases in the hypotensive effect of neurotensin. At the highest dose of neurotensin (3000 µM), MAP fell to −67 ± 2 mmHg from baseline (Figure 1B, P < 0.001). The IC50 calculated from the MAP log-dose–response curve was 13 µM (Figure 1C).
Figure 1.
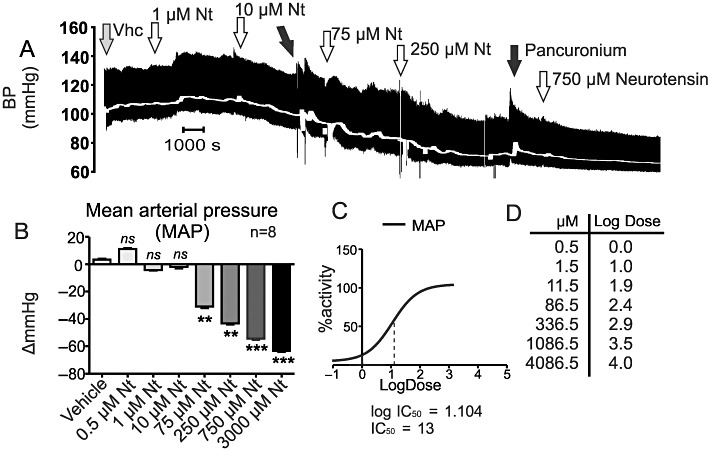
Effects of vehicle or neurotensin on blood pressure (BP), and MAP following i.t. administration at T6. (A) An example of cumulative neurotensin dose–response (doses from 1 to 750 µM are shown). (B) Grouped data of the cumulative effect of neurotensin on MAP. Neurotensin concentrations are compared with control. (C and D) Dose–response curve and log-dose values corresponding to the cumulative concentrations of neurotensin. ns, non-significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Arterial blood gas parameters were recorded and, with the exception of PaO2, were within the normal range: pH = 7.37 ± 0.01, PaCO2= 39 ± 2 mmHg, PaO2 > 200 mmHg, HCO3- 21 ± 1 mEq·L−1 and anion gap 14 ± 1 mEq·L−1.
Hypotension following administration of 75 and 3000 µM neurotensin
In the second group of animals, 75 µM followed by 3000 µM neurotensin was administered (Figure 2A and 2B). The hypotensive effects of neurotensin averaged −12 ± 2 mmHg (P < 0.01; Figure 2A and 2C) and −25 ± 5 mmHg (P < 0.01; Figure 2A and 2C), for 75 and 3000 µM, respectively (Figure 4). Both effects were significant, as was the difference between the two doses (Figure 4).
Figure 2.
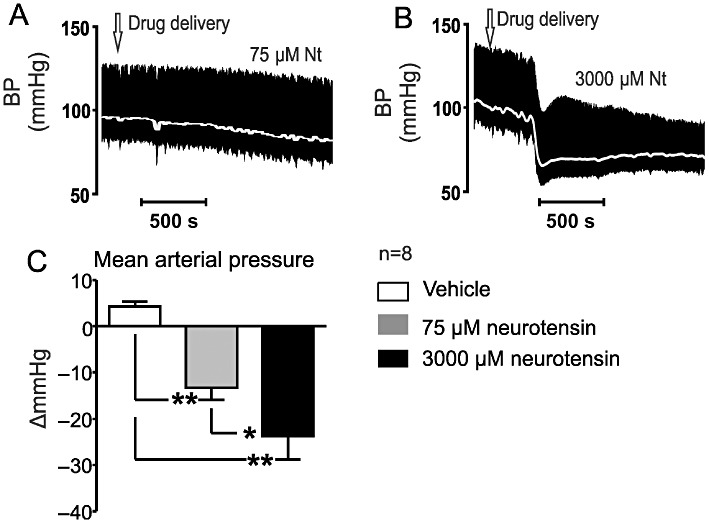
(A and B) Effects of i.t. applied neurotensin (75 and 3000 µM) on MAP (grey; pulsatile arterial pressure, black). (C) Grouped data of i.t. applied neurotensin (75 and 3000 µM) on MAP. *P < 0.01, **P < 0.001.
Figure 4.
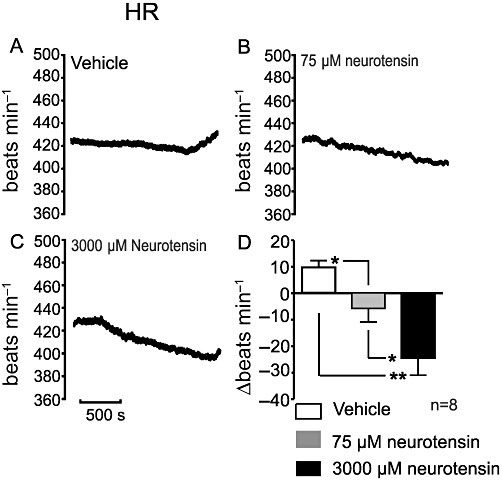
(A–C) Effects of i.t. administration of vehicle or neurotensin (75 and 3000 µM) on HR (recording). (D) Grouped data show the effects of i.t. administered vehicle and neurotensin on HR. *P < 0.05, **P < 0.01.
Arterial blood gas parameters were also measured in this group of animals: pH = 7.39 ± 0.01, PaCO2= 37 ± 1 mmHg, PaO2 > 200 mmHg, HCO3- 21 ± 1 mEq·L−1 and anion gap 13 ± 1 mEq·L−1.
Sympathoinhibitory and bradycardic effects of 75 and 3000 µM neurotensin
sSNA was dose-dependently inhibited following i.t. administration of neurotensin (Figure 3). sSNA inhibition fell from 22 ± 3% to −2 ± 5%, following administration of 75 µM (P < 0.05, Figure 3B and 3D). In the case of 3000 µM neurotensin, sSNA was reduced to −26 ± 9% (P < 0.01; Figure 3C and 3D). The difference between the two neurotensin doses was also significant (P < 0.05). HR, cardiac contractility and ECG parameters were also assessed following application of 75 and 3000 µM neurotensin. HR decreased from 11 ± 2 to −7 ± 5 beats min−1 (75 µM neurotensin; P < 0.05, Figure 4A, B and D) and −26 ± 6 beats min−1 (3000 µM neurotensin; P < 0.01, Figure 4A, C and D). A dose-dependent difference was noted between 75 and 3000 µM neurotensin (P < 0.05). Cardiac contractility was reduced dose-dependently from 2900 ± 200 mmHg·s−1 to an average of 2200 ± 170 mmHg·s−1 (75 µM neurotensin, P < 0.05; Figure 5A) and 2100 ± 160 mmHg·s−1 (3000 µM neurotensin, P < 0.05; Figure 5A). Evaluation of ECG time parameters revealed a dose-dependent prolongation of the PR interval (75 µM = 1.3 ± 0.3 ms, P < 0.05; 3000 µM = 2.5 ± 0.4 ms, P < 0.01; Figure 5B) and ST segment (75 µM = 1.8 ± 0.2 ms, P < 0.05; 3000 µM = 2.0 ± 0.2 ms, P < 0.05; Figure 5C). The QRS segment was not altered.
Figure 3.
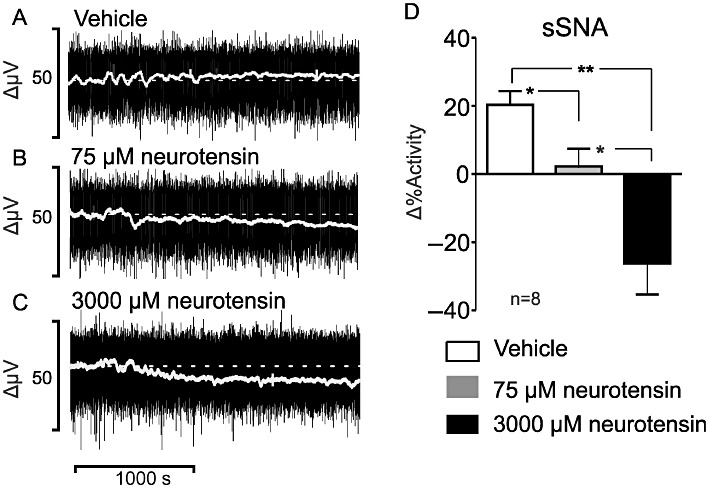
(A–C) Effects of i.t.. administration of vehicle or neurotensin (75 and 3000 µM) on sSNA (raw recording, black; integrated, grey). (D) Grouped data summarizing the effects of i.t. applied vehicle and neurotensin on sSNA. *P < 0.05, **P < 0.01.
Figure 5.
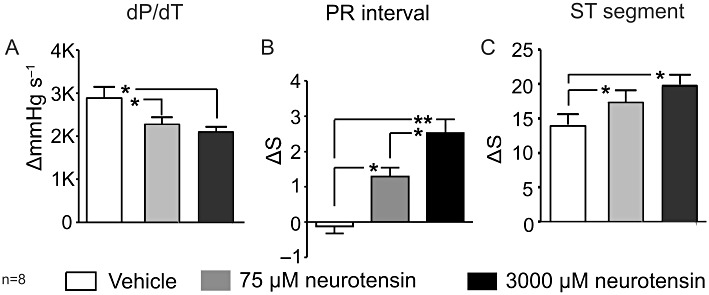
Grouped data summarizing the effects of 75 and 3000 µM neurotensin on (A) cardiac contractility (dP/dT), (B) PR interval and (C) ST segment. *P < 0.05, **P < 0.01.
Effect on phrenic nerve discharge
PNamp decreased markedly following 75 and 3000 µM neurotensin; it was reduced from 76 ± 5% to 56 ± 2% (P < 0.05; Figure 6A) and from 76 ± 5% to 20 ± 5% (P < 0.01; Figure 6A), respectively. The difference between the effects of the two neurotensin doses was also significant (P < 0.01; Figure 6A).
Figure 6.
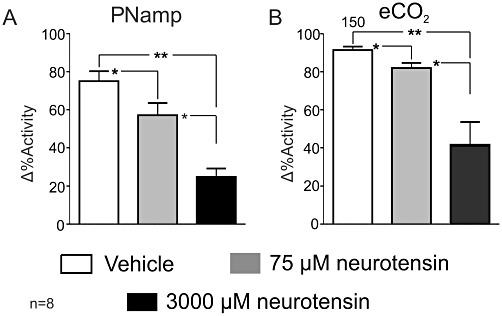
Grouped data showing the effects of 75 and 3000 µM neurotensin on (A) PNamp and (B) etCO2. *P < 0.05, **P < 0.01.
There was no change in PNf at either dose. etCO2 decreased dose-dependently by 75 µM from 0.17 ± 0.02% to −0.3 ± 0.07% (P < 0.05; Figure 6B). etCO2 fell from 0.17 ± 2% to −0.93 ± 0.17% following administration of 3000 µM neurotensin (P < 0.01; Figure 6B). Finally, the reduction in etCO2 was also greater in the case of the 3000 to 75 µM neurotensin, −0.6 ± 9% (P < 0.05; Figure 6B).
I.t. neurotensin increases the sensitivity of the sympathetic baroreflex
Rapid changes in sSNA in relation to blood pressure following administration of nitroprusside and phenylephrine were plotted and fitted to a sigmoid curve. Neurotensin increased the range of the baroreflex curve and shifted the curve upwards and slightly to the right (Figure 7). The upper plateau was increased from 119 ± 12% to 172 ± 14% following 75 µM (Table 1; P < 0.001) and from 119 ± 12% to 165 ± 11% following 3000 µM (Table 1, P < 0.01). The lower plateau of sSNA was reduced by 3000 µM, but not by 75 µM, neurotensin (Table 1). Overall, the sympathetic baroreflex range was increased by 51 ± 12% (75 µM, P < 0.01; Table 1) and 67 ± 15% (3000 µM, P < 0.01; Table 1). The threshold (192 ± 13 mmHg, P < 0.01; Table 2) and blood pressure range (110 ± 15 mmHg) were also increased following 3000 µM neurotensin (Table 2). Finally, the gain of the sympathetic baroreflex curve was enhanced markedly from −1.8 ± 0.4 normalized arbitrary units (au) mmHg−1 to −2.5 ± 0.5 normalized arbitrary units mmHg−1 (75 mM, P < 0.01; Table 2) and from −1.8 ± 0.4 normalized arbitrary units mmHg−1 to −2.3 ± 0.4 normalized arbitrary units mmHg−1 (3000 µM, P < 0.05; Table 2).
Figure 7.
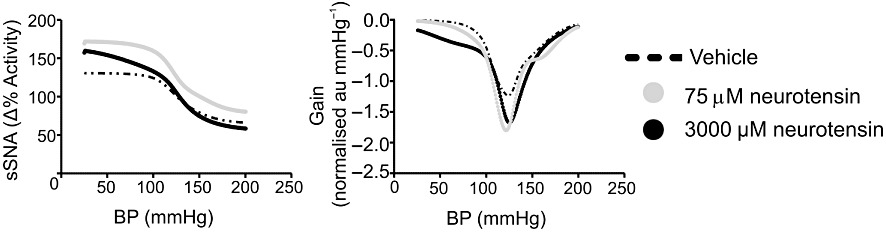
Sigmoid curves for vehicle and neurotensin (75 µM and 3000 µM) responses to sympathetic baroreflex activation.
Table 1.
Grouped data for vehicle and neurotensin responses to sympathetic baroreflex activation
| Midpoint (mmHg) | Upper plateau (%baseline) | Lower plateau (%baseline) | Range of sSNA (%baseline) | |
|---|---|---|---|---|
| Vehicle | 128 ± 5 | 119 ± 12 | 76 ± 11 | 43 ± 18 |
| 75 µM neurotensin | 126 ± 5 | 175 ± 14** | 79 ± 13 | 96 ± 12** |
| 3000 µM neurotensin | 117 ± 8 | 165 ± 11** | 56 ± 9* | 109 ± 15** |
Values are means ± SEM.
P < 0.05,
P < 0.01.
Table 2.
Grouped data for vehicle and neurotensin responses to sympathetic baroreflex activation
| Max gain (norm·au mmHg−1) | Saturation (mmHg) | Threshold (mmHg) | Range of BP (mmHg) | |
|---|---|---|---|---|
| Vehicle | −1.8 ± 0.4 | 77 ± 8 | 116 ± 11 | 39 ± 6 |
| 75 µM neurotensin | −2.5 ± 0.5** | 71 ± 11** | 174 ± 13 | 103 ± 8** |
| 3000 µM neurotensin | −2.3 ± 0.4* | 42 ± 16** | 192 ± 13** | 150 ± 21** |
Values are means ± SEM. Maximum gain is the steepest slope of the sigmoid curve.
P < 0.05,
P < 0.01.
BP, blood pressure.
Neurotensin reduces MAP response during hypercarbia (central chemoreflex)
The actions of 75 and 3000 µM neurotensin on MAP, HR, sSNA and PNamp were measured during hyperoxic hypercapnic stimuli (10% CO2 in O2 for 90 s). Neurotensin caused an attenuation of the hypertensive effect on hypercarbia. After administration of neurotensin, MAP was decreased by −3 ± 1 mmHg (75 µM, P < 0.05) and −4 ± 1 mmHg (3000 µM, P < 0.01) compared with −11 ± 2 before neurotensin. The HR, PNamp and sSNA responses to hypercarbia were unaffected by either dose of neurotensin.
I.t. SR 142948A causes hypotension, sympathoinhibition and central bradypnoea
SR 149248A (5 mM; an antagonist at both the NTS1 and NTS2 receptors) caused a significant decrease in MAP (Figure 8A-1–4). Hypotension was greater when SR 149248A was administered alone (−35 ± 7 mmHg; P < 0.01, Figure 8A-4) than when in combination with 75 µM neurotensin (−22 ± 5 mmHg; P < 0.05; Figure 8A-4). The difference in MAP between SR 142948A and 75 µM neurotensin (−12 ± 2 mmHg) was also significantly greater (P < 0.05; Figure 8A-4). MAP values returned to baseline approximately 30 min following i.t. administration of SR 149248A. sSNA was decreased significantly by SR 149248A to a mean value of −44 ± 9% (P < 0.01; Figure 8B-1–2) and to −72 ± 14% (SR 142948A + 75 µM neurotensin, P < 0.01; Figure 8B-2). As with MAP, sSNA returned to baseline approximately 30 min following i.t. administration of SR 149248A. A significant bradycardia was observed following SR 142948A alone (−54 ± 14 bpm; P < 0.05, Figure 8C) and following SR 142948A + 75 µM neurotensin (−42 ± 5 bpm; P < 0.01, Figure 8C). SR 142948A reduced cardiac contractility from 3500 ± 200 to 2700 ± 300 mmHg·s−1 (P < 0.05, Figure 8D). PNamp was reduced by SR 142948A independently and when given in combination with 75 µM neurotensin (Figure 8E). PNamp fell from 75 ± 5% to 26 ± 6% (P < 0.01; Figure 8F) and from 75 ± 5% to 37 ± 4%, respectively (P < 0.01; Figure 8F). A decrease in the frequency of phrenic nerve discharge was observed following administration of SR 142948A alone (P < 0.01, Figure 8G) and in combination with 75 µM neurotensin (P < 0.05, Figure 8G). Finally, SR 142948A caused a fall in etCO2 from −0.11 ± 0.06% to −0.38 ± 0.05% (P < 0.01), while SR 142948A given together with 75 µM neurotensin decreased etCO2 from −0.11 ± 0.06% to −0.41 ± 0.06% (P < 0.01).
Figure 8.
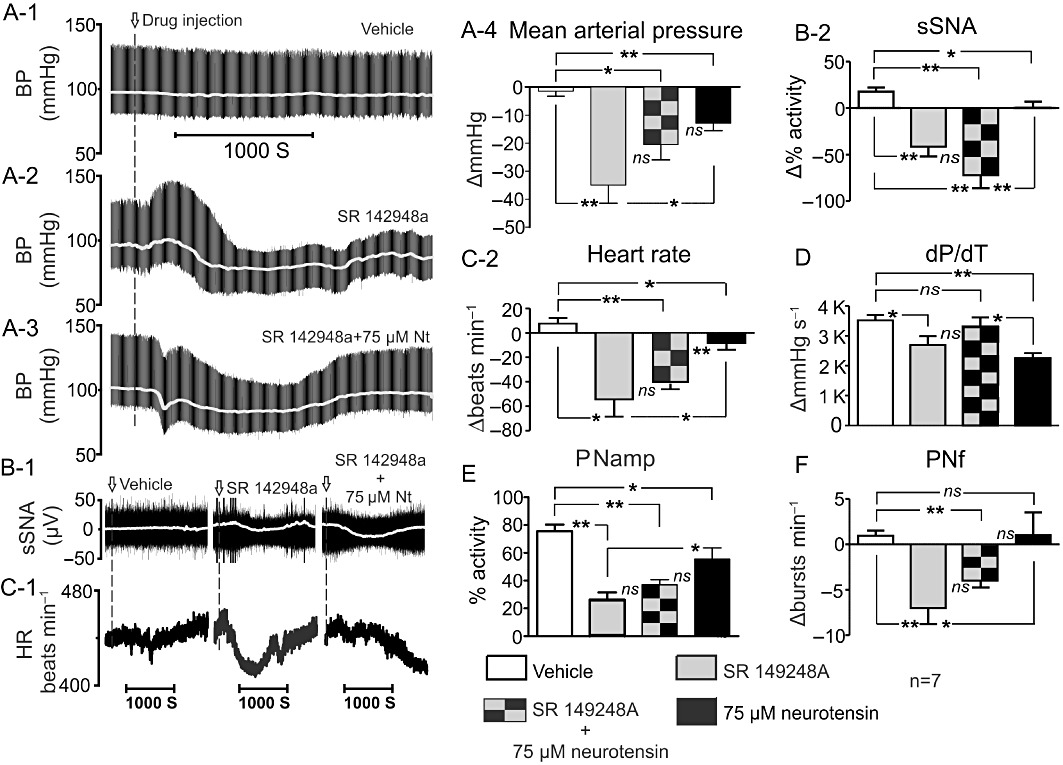
(A-1) Effect of intrathecally applied vehicle, (A-2) SR 142948A or (A-3) SR 142948A + neurotensin (75 µM) on blood pressure (MAP, grey, pulsatile arterial pressure, black). (A-4) Grouped data of the effects of vehicle, SR 142948A, SR 142948A + neurotensin (75 µM) and neurotensin (75 µM) on MAP. (B-1) sSNA (raw, black, integrated sSNA, grey). (B-2) Grouped data sSNA. (C-1) HR and C-2 Grouped data HR. (D) Cardiac contractility (dP/dT), (E) PNamp and (G) PNf. ns, non significant, *P < 0.05, **P < 0.01.
Arterial blood gas parameters were measured and averaged: pH = 7.42 ± 0.01, PaCO2= 38 ± 1 mmHg, PaO2 > 200 mmHg, HCO3- 23 ± 1 mEq·L−1 and anion gap 13 ± 1 mEq·L−1.
Discussion and conclusions
The main findings of this study are as follows: firstly, neurotensin dose-dependently decreased MAP when applied i.t. at T6. Secondly, administration of 75 and 3000 µM neurotensin, in a separate set of animals, caused a dose-related hypotension, sympathoinhibition, bradycardia and a decrease in PNamp. Thirdly, baroreflex sensitivity was increased following administration of 75 and 3000 µM neurotensin. Finally, SR 142948A caused a neurotensin-like response that included hypotension, sympathoinhibition, bradycardia and a decrease in central inspiratory drive.
Autonomic nuclei in the ventrolateral medulla are essential for the central regulation of circulation and breathing (Pilowsky and Goodchild, 2002; Guyenet, 2006; Smith et al., 2009; Guyenet et al., 2010a). Rapid changes in blood pressure (Pilowsky and Goodchild, 2002; Pilowsky et al., 2009) or blood pH (Abbott et al., 2009; Guyenet et al., 2010b; Spirovski et al., 2011; Takakura et al., 2011) are controlled by barosensitive and chemosensitive neurons in the brainstem. Recent studies demonstrate the importance of neuropeptides such as bombesin (Zogovic and Pilowsky, 2011) and somatostatin (Burke et al., 2008) in cardiorespiratory neurotransmission.
In mammals, NTS1, NTS2 and NTS3 are neurotensin-specific receptors (Vincent, 1995; Mazella and Vincent, 2006). NTS1 and NTS2 are seven-transmembrane receptors coupled to Gq/G11 (Mazella et al., 1996; Gailly, 1998; Gailly et al., 2000). NTS3/sortilin 1 is a single-domain receptor that binds promiscuously to many ligands including brain derived neurotrophic factor, neurotensin and lipoprotein lipase (Mazella et al., 1998; Nielsen et al., 1999; 2001). Neurotensin and neurotensin receptors are found in many autonomic sites including: the nucleus of the solitary tract, vagus nerve, the paraventricular hypothalamic nucleus and pontine nuclei (Jennes et al., 1982; Mai et al., 1987; Boudin et al., 1996; Rostene and Alexander, 1997; Fassio et al., 2000; Binder et al., 2001; Sarrieau et al., 1985; Sarret et al., 2003). In human, NTS1 and NTS2 are found in the spinal grey matter, with highest density in the dorsal horns, and lowest in the ventral horns (Faull et al., 1989). NTS3 gene and protein are also expressed in the spinal cord (Petersen et al., 1997; Hermans-Borgmeyer et al., 1998). In cat, neurotensin-like immunoreactivity co-exists with somatostatin, and substance P, in sympathetic pre-ganglionic neurons (Krukoff, 1987). In rat, neurotensin-immunoreactive axons are found in dorsal horn (Ninkovic et al., 1981; Todd et al., 1992; 1994). I.t. neurotensin causes analgesia and hypothermia in rat (Martin and Naruse, 1982; Spampinato et al., 1988). Altogether, the results suggest that neurotensin can affect spinal sensory and autonomic pathways to influence phrenic and sympathetic nerve activity.
In the current study, i.t. neurotensin caused a gradual dose-dependent hypotension evident approximately 5 min following administration. In previous studies, neurotensin application in the nucleus of the solitary tract (Ciriello and Zhang, 1997) or the lateral ventricles (Rioux et al., 1981) caused a prompt effect on blood pressure. The discrepancy may be a property of the pharmacokinetics of neurotensin and the site of administration. Tachyphylaxis was also observed in these experiments, as previously reported following i.v. neurotensin administration in rat (Rioux et al., 1981; Di Paola and Richelson, 1990). Administration of higher concentrations of neurotensin after the threshold dose (75 µM) resulted in a further increase in the hypotensive effect, but the maximum effect of each higher neurotensin dose (250–3000 µM) was lower than the maximum effect of 75 µM neurotensin. For this reason, 75 and 3000 µM neurotensin were administered in separate groups of animals and caused a true dose-dependent hypotension, sympathoinhibition and bradycardia. In the current study, baroreflex sensitivity was enhanced in the case of both 75 and 3000 µM neurotensin. Similar observations were reported following neurotensin microinjection into the nucleus of the solitary tract (Kubo and Kihara, 1990). In contrast to the study by Kubo and Kihara (1990), the study by Chen et al. (1990), which differed in rat strains, type of anaesthetic and mode of triggering of the baroreflex, suggested a suppression of barosensitivity. Neurotensin only attenuated the pressor responses in MAP to acute hypercapnic hyperoxia, but not PNamp, sSNA and HR.
I.t. neurotensin reduced the amplitude of phrenic nerve discharge, probably by activating spinobulbar pathways that act upon respiratory pattern generators in the brainstem. Postmortem verification of the injection site showed dye spread of only few segments rostro-caudally from the tip of the i.t. catheter, as previously documented (Yaksh and Rudy, 1976). In addition, spinal anaesthesia (Shahid et al., 2011) or transection (Rahman et al., 2011) at C8 completely inhibits the effects on PNamp induced by i.t. drugs delivered at thoracic spinal segments.
SR 142948A is a synthetic NTS1 and NTS2 receptor antagonist of many neurotensin actions (Gully et al., 1997; Schaeffer et al., 1998). SR142948A is more effective at preventing neurotensin-induced hypotension than SR48692 (Schaeffer et al., 1998), an antagonist that preferentially binds NTS1 but is an agonist at NTS2 (Gully et al., 1996; Botto et al., 1997). For these reasons, SR 142948A was chosen to determine if the actions of i.t. neurotensin could be prevented, or if SR 142948A has any effects on its own, that might suggest a tonic release of neurotensin. In a previous study, pretreatment with oral SR 142948A prevented the hypotensive effects of i.v. neurotensin in anaesthetized rats but had no effects of its own (Schaeffer et al., 1998). Here, we found that pretreatment with i.t. SR 142948A is profoundly hypotensive (∼35 mmHg). This finding suggests agonist effects of SR 142948A in the spinal cord since the actions of SR 142948A were similar to the actions of neurotensin. The hypotension developed gradually, reaching a trough after ∼15 min and returned to baseline around 30 min following administration. The effect of SR 142948A on MAP was greater than the effects caused by SR 142948A given together with 75 µM neurotensin. Although the hypotensive effects on MAP were unexpected, they were not entirely surprising. SR 142948A is known to act as an agonist on CHO cells transfected with NTS2 (Vita et al., 1998). The MAP effects of SR 142948A were accompanied by a sympathoinhibition. Interestingly, the sympathoinhibition that followed administration of SR 142948A together with 75 µM neurotensin was synergistic. The sympathoinhibitory effects were associated with a reduction in HR and contractility, as well as central inspiratory inhibition. Effects on PNf were presumably mediated by actions on spinobulbar pathways since PNf changes cannot be explained by a direct action on phrenic motoneurons.
The observed actions of SR142948A are probably a result of combined effect of route of administration and agonist effects. It is not likely that the pharmacokinetics of SR142948A play a crucial role in the observed actions. In contrast to the short half-life of neurotensin (Checler et al., 1986; Barelli et al., 1994), SR142948A inactivation does not happen immediately (Gully et al., 1997; Schaeffer et al., 1998; Casti et al., 2004). Nevertheless, in this study, SR142948A was delivered i.t. so as to act directly and avoid immediate sequestration/inactivation. The mechanisms underlying the actions of SR142948A are speculative. In the periphery, it was suggested that effects of neurotensin or SR142948A were a result of activation, or inhibition of the release of histamine in mast cells via specific neurotensin receptors respectively (Schaeffer et al., 1998). I.t. SR142948A might act, as a direct agonist at NTS1 and/or NTS2 or the hypotensive effects might be a result of heterodimerization between NTS1 and NTS2 in the presence of SR142948A. In particular, it was shown that in COS-7 transfected cells NTS2 influences the intracellular trafficking and membrane expression of NTS1 (Perron et al., 2007). Finally, an interaction of SR142948A with the NTS3/sortilin 1 receptor or other neurotensin receptor subtype, such as the recently identified fourth neurotensin receptor (Li et al., 2005), in the spinal cord cannot be excluded.
In summary, we showed that i.t. neurotensin causes dose-dependent hypotension and sympathoinhibition. I.t. neurotensin affects central inspiratory drive via a reduction in the amplitude of phrenic nerve. I.t. SR 142948A does not antagonize the actions of neurotensin but elicits neurotensin-like effects that cause hypotension, sympathoinhibition, bradycardia, a reduction in cardiac contractility and central respiratory drive. Taken together, these findings demonstrate an important role for neurotensin receptors in the CNS tonic and reflex regulation of central cardiorespiratory control mechanisms.
Acknowledgments
The study was supported by the National Health and Medical Research Council of Australia (457069, 457080 and 604002), Australian Research Council (DP110102110), Macquarie University and the Garnett Passe and Rodney Williams Memorial Foundation. BZ is supported by a Macquarie Research Excellence Scholarship. The authors are grateful to Tara Bautista, Peter Burke, Eyitemi Egwuenu, Willian Korim, Simon McMullan and Darko Spirovski, for their advice.
Glossary
- etCO2
end-tidal carbon dioxide
- HR
heart rate
- i.t
intrathecal
- MAP
mean arterial pressure
- NTS1
neurotensin receptor 1
- NTS2
neurotensin receptor 2
- NTS3
neurotensin receptor 3
- PNamp
phrenic nerve amplitude
- PNf
phrenic nerve frequency
- SR
142948A, 2-[[5-(2,6-dimethoxyphenyl)-1-(4-(N-(3-dimethylaminopropyl)-N-methylcarbamoyl)-2-isopropylphenyl)-1H-pyrazole3-carbonyl]amino] adamantane-2-carboxylic acid, hydrochloride
- sSNA
splanchnic sympathetic nerve activity
- T6
sixth thoracic spinal cord segment
Conflicts of interest
None.
References
- Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, et al. Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci. 2009;29:5806–5819. doi: 10.1523/JNEUROSCI.1106-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barelli H, Fox-Threlkeld JE, Dive V, Daniel EE, Vincent JP, Checler F. Role of endopeptidase 3.4.24.16 in the catabolism of neurotensin, in vivo, in the vascularly perfused dog ileum. Br J Pharmacol. 1994;112:127–132. doi: 10.1111/j.1476-5381.1994.tb13041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev. 2001;53:453–486. [PubMed] [Google Scholar]
- Botto JM, Guillemare E, Vincent JP, Mazella J. Effects of SR 48692 on neurotensin-induced calcium-activated chloride currents in the Xenopus oocyte expression system: agonist-like activity on the levocabastine-sensitive neurotensin receptor and absence of antagonist effect on the levocabastine insensitive neurotensin receptor. Neurosci Lett. 1997;223:193–196. doi: 10.1016/s0304-3940(97)13437-1. [DOI] [PubMed] [Google Scholar]
- Boudin H, Pelaprat D, Rostene W, Beaudet A. Cellular distribution of neurotensin receptors in rat brain: immunohistochemical study using an antipeptide antibody against the cloned high affinity receptor. J Comp Neurol. 1996;373:76–89. doi: 10.1002/(SICI)1096-9861(19960909)373:1<76::AID-CNE7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Burke PG, Li Q, Costin ML, McMullan S, Pilowsky PM, Goodchild AK. Somatostatin 2A receptor-expressing presympathetic neurons in the rostral ventrolateral medulla maintain blood pressure. Hypertension. 2008;52:1127–1133. doi: 10.1161/HYPERTENSIONAHA.108.118224. [DOI] [PubMed] [Google Scholar]
- Carraway R, Leeman SE. The isolation of a new hypotensive peptide, neurotensin, from bovine hypothalami. J Biol Chem. 1973;248:6854–6861. [PubMed] [Google Scholar]
- Carraway R, Ruane SE, Kim HR. Distribution and immunochemical character of neurotensin-like material in representative vertebrates and invertebrates: apparent conservation of the COOH-terminal region during evolution. Peptides. 1982;3:115–123. doi: 10.1016/0196-9781(82)90038-9. [DOI] [PubMed] [Google Scholar]
- Casti P, Marchese G, Casu G, Ruiu S, Pani L. Blockade of neurotensin receptors affects differently hypo-locomotion and catalepsy induced by haloperidol in mice. Neuropharmacology. 2004;47:128–135. doi: 10.1016/j.neuropharm.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Checler F, Vincent JP, Kitabgi P. Neuromedin N: high affinity interaction with brain neurotensin receptors and rapid inactivation by brain synaptic peptidases. Eur J Pharmacol. 1986;126:239–244. doi: 10.1016/0014-2999(86)90053-1. [DOI] [PubMed] [Google Scholar]
- Chen CT, Chan JY, Barnes CD, Chan SH. Tonic suppression of baroreceptor reflex by endogenous neurotensin in the rat. Regul Pept. 1990;28:23–37. doi: 10.1016/0167-0115(90)90061-z. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- Ciriello J, Zhang TX. Cardiovascular effects of neurotensin microinjections into the nucleus of the solitary tract. Brain Res. 1997;749:35–43. doi: 10.1016/s0006-8993(96)01176-6. [DOI] [PubMed] [Google Scholar]
- Di Paola ED, Richelson E. Cardiovascular effects of neurotensin and some analogues on rats. Eur J Pharmacol. 1990;175:279–283. doi: 10.1016/0014-2999(90)90565-n. [DOI] [PubMed] [Google Scholar]
- DiBona GF. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 2002;11:197–200. doi: 10.1097/00041552-200203000-00011. [DOI] [PubMed] [Google Scholar]
- Dubuc I, Sarret P, Labbe-Jullie C, Botto JM, Honore E, Bourdel E, et al. Identification of the receptor subtype involved in the analgesic effect of neurotensin. J Neurosci. 1999;19:503–510. doi: 10.1523/JNEUROSCI.19-01-00503.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassio A, Evans G, Grisshammer R, Bolam JP, Mimmack M, Emson PC. Distribution of the neurotensin receptor NTS1 in the rat CNS studied using an amino-terminal directed antibody. Neuropharmacology. 2000;39:1430–1442. doi: 10.1016/s0028-3908(00)00060-5. [DOI] [PubMed] [Google Scholar]
- Faull RL, Villiger JW, Dragunow M. Neurotensin receptors in the human spinal cord: a quantitative autoradiographic study. Neuroscience. 1989;29:603–613. doi: 10.1016/0306-4522(89)90134-6. [DOI] [PubMed] [Google Scholar]
- Gailly P. Ca2+ entry in CHO cells, after Ca2+ stores depletion, is mediated by arachidonic acid. Cell Calcium. 1998;24:293–304. doi: 10.1016/s0143-4160(98)90053-7. [DOI] [PubMed] [Google Scholar]
- Gailly P, Najimi M, Hermans E. Evidence for the dual coupling of the rat neurotensin receptor with pertussis toxin-sensitive and insensitive G-proteins. FEBS Lett. 2000;483:109–113. doi: 10.1016/s0014-5793(00)02095-0. [DOI] [PubMed] [Google Scholar]
- Goedert M, Lightman SL, Emson PC. Neurotensin in the rat anterior pituitary gland: effects of endocrinological manipulations. Brain Res. 1984;299:160–163. doi: 10.1016/0006-8993(84)90800-x. [DOI] [PubMed] [Google Scholar]
- Gully D, Lespy L, Canton M, Rostene W, Kitabgi P, Le Fur G, et al. Effect of the neurotensin receptor antagonist SR48692 on rat blood pressure modulation by neurotensin. Life Sci. 1996;58:665–674. doi: 10.1016/s0024-3205(96)80005-1. [DOI] [PubMed] [Google Scholar]
- Gully D, Labeeuw B, Boigegrain R, Oury-Donat F, Bachy A, Poncelet M, et al. Biochemical and pharmacological activities of SR 142948A, a new potent neurotensin receptor antagonist. J Pharmacol Exp Ther. 1997;280:802–812. [PubMed] [Google Scholar]
- Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Abbott SB, Depuy SD, Fortuna MG, Kanbar R. Central CO2 chemoreception and integrated neural mechanisms of cardiovascular and respiratory control. J Appl Physiol. 2010a;108:995–1002. doi: 10.1152/japplphysiol.00712.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bayliss DA. Central respiratory chemoreception. J Comp Neurol. 2010b;518:3883–3906. doi: 10.1002/cne.22435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton AC. Blood pressure control – special role of the kidneys and body fluids. Science. 1991;252:1813–1816. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- Hall JE. The kidney, hypertension, and obesity. Hypertension. 2003;41:625–633. doi: 10.1161/01.HYP.0000052314.95497.78. [DOI] [PubMed] [Google Scholar]
- Hermans-Borgmeyer I, Hampe W, Schinke B, Methner A, Nykjaer A, Susens U, et al. Unique expression pattern of a novel mosaic receptor in the developing cerebral cortex. Mech Dev. 1998;70:65–76. doi: 10.1016/s0925-4773(97)00177-9. [DOI] [PubMed] [Google Scholar]
- Ifuku H, Taniguchi K, Matsumoto H. Noninvasive assessment of cardiac contractility by using (dP/dt)/P of carotid artery pulses during exercise. Eur J Appl Physiol Occup Physiol. 1994;69:244–249. doi: 10.1007/BF01094796. [DOI] [PubMed] [Google Scholar]
- Jennes L, Stumpf WE, Kalivas PW. Neurotensin: topographical distribution in rat brain by immunohistochemistry. J Comp Neurol. 1982;210:211–224. doi: 10.1002/cne.902100302. [DOI] [PubMed] [Google Scholar]
- Katholi RE, Rocha-Singh KJ. The role of renal sympathetic nerves in hypertension: has percutaneous renal denervation refocused attention on their clinical significance? Prog Cardiovasc Dis. 2009;52:243–248. doi: 10.1016/j.pcad.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kislauskis E, Bullock B, McNeil S, Dobner PR. The rat gene encoding neurotensin and neuromedin N. Structure, tissue-specific expression, and evolution of exon sequences. J Biol Chem. 1988;263:4963–4968. [PubMed] [Google Scholar]
- Krukoff TL. Coexistence of neuropeptides in sympathetic preganglionic neurons of the cat. Peptides. 1987;8:109–112. doi: 10.1016/0196-9781(87)90172-0. [DOI] [PubMed] [Google Scholar]
- Kubo T, Kihara M. Modulation of the aortic baroreceptor reflex by neuropeptide Y, neurotensin and vasopressin microinjected into the nucleus tractus solitarii of the rat. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:182–188. doi: 10.1007/BF00166962. [DOI] [PubMed] [Google Scholar]
- Li JH, Sicard F, Salam MA, Baek M, LePrince J, Vaudry H, et al. Molecular cloning and functional characterization of a type-I neurotensin receptor (NTR) and a novel NTR from the bullfrog brain. J Mol Endocrinol. 2005;34:793–807. doi: 10.1677/jme.1.01709. [DOI] [PubMed] [Google Scholar]
- Mai JK, Triepel J, Metz J. Neurotensin in the human brain. Neuroscience. 1987;22:499–524. doi: 10.1016/0306-4522(87)90349-6. [DOI] [PubMed] [Google Scholar]
- Martin GE, Naruse T. Differences in the pharmacological actions of intrathecally administered neurotensin and morphine. Regul Pept. 1982;3:97–103. doi: 10.1016/0167-0115(82)90086-6. [DOI] [PubMed] [Google Scholar]
- Mazella J, Vincent JP. Functional roles of the NTS2 and NTS3 receptors. Peptides. 2006;27:2469–2475. doi: 10.1016/j.peptides.2006.04.026. [DOI] [PubMed] [Google Scholar]
- Mazella J, Botto JM, Guillemare E, Coppola T, Sarret P, Vincent JP. Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain. J Neurosci. 1996;16:5613–5620. doi: 10.1523/JNEUROSCI.16-18-05613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazella J, Zsurger N, Navarro V, Chabry J, Kaghad M, Caput D, et al. The 100-kDa neurotensin receptor is gp95/sortilin, a non-G-protein-coupled receptor. J Biol Chem. 1998;273:26273–26276. doi: 10.1074/jbc.273.41.26273. [DOI] [PubMed] [Google Scholar]
- Mule F, Serio R, Postorino A, Vetri T, Bonvissuto F. Antagonism by SR 48692 of mechanical responses to neurotensin in rat intestine. Br J Pharmacol. 1996;117:488–492. doi: 10.1111/j.1476-5381.1996.tb15216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MS, Jacobsen C, Olivecrona G, Gliemann J, Petersen CM. Sortilin/neurotensin receptor-3 binds and mediates degradation of lipoprotein lipase. J Biol Chem. 1999;274:8832–8836. doi: 10.1074/jbc.274.13.8832. [DOI] [PubMed] [Google Scholar]
- Nielsen MS, Madsen P, Christensen EI, Nykjaer A, Gliemann J, Kasper D, et al. The sortilin cytoplasmic tail conveys Golgi-endosome transport and binds the VHS domain of the GGA2 sorting protein. EMBO J. 2001;20:2180–2190. doi: 10.1093/emboj/20.9.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog Neurobiol. 2002;67:53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Ninkovic M, Hunt SP, Kelly JS. Effect of dorsal rhizotomy on the autoradiographic distribution of opiate and neurotensin receptors and neurotensin-like immunoreactivity within the rat spinal cord. Brain Res. 1981;230:111–119. doi: 10.1016/0006-8993(81)90395-4. [DOI] [PubMed] [Google Scholar]
- Osumi Y, Nagasaka Y, Wang FLH, Fujiwara M. Inhibition of gastric acid secretion and mucosal blood flow induced by intraventricularly applied neurotensin in rats. Life Sci. 1978;23:2275–2280. doi: 10.1016/0024-3205(78)90192-3. [DOI] [PubMed] [Google Scholar]
- Perron A, Sharif N, Sarret P, Stroh T, Beaudet A. NTS2 modulates the intracellular distribution and trafficking of NTS1 via heterodimerization. Biochem Biophys Res Commun. 2007;353:582–590. doi: 10.1016/j.bbrc.2006.12.062. [DOI] [PubMed] [Google Scholar]
- Petersen CM, Nielsen MS, Nykjaer A, Jacobsen L, Tommerup N, Rasmussen HH, et al. Molecular identification of a novel candidate sorting receptor purified from human brain by receptor-associated protein affinity chromatography. J Biol Chem. 1997;272:3599–3605. doi: 10.1074/jbc.272.6.3599. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Pilowsky PM, Lung MS, Spirovski D, McMullan S. Differential regulation of the central neural cardiorespiratory system by metabotropic neurotransmitters. Philos Trans R Soc Lond B Biol Sci. 2009;364:2537–2552. doi: 10.1098/rstb.2009.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman AA, Shahid IZ, Pilowsky PM. Intrathecal neuromedin U induces biphasic effects on sympathetic vasomotor tone, increases respiratory drive and attenuates sympathetic reflexes in rat. Br J Pharmacol. 2011;164:617–631. doi: 10.1111/j.1476-5381.2011.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioux F, Quirion RS, Pierre S, Regoli D, Jolicoeur FB, Belanger F, et al. The hypotensive effect of centrally administered neurotensin in rats. Eur J Pharmacol. 1981;69:241–247. doi: 10.1016/0014-2999(81)90469-6. [DOI] [PubMed] [Google Scholar]
- Rostene WH, Alexander MJ. Neurotensin and neuroendocrine regulation. Front Neuroendocrinol. 1997;18:115–173. doi: 10.1006/frne.1996.0146. [DOI] [PubMed] [Google Scholar]
- Roussy G, Dansereau MA, Baudisson S, Ezzoubaa F, Belleville K, Beaudet N, et al. Evidence for a role of NTS2 receptors in the modulation of tonic pain sensitivity. Mol Pain. 2009;5:38. doi: 10.1186/1744-8069-5-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarret P, Krzywkowski P, Segal L, Nielsen MS, Petersen CM, Mazella J, et al. Distribution of NTS3 receptor/sortilin mRNA and protein in the rat central nervous system. J Comp Neurol. 2003;461:483–505. doi: 10.1002/cne.10708. [DOI] [PubMed] [Google Scholar]
- Sarret P, Esdaile MJ, Perron A, Martinez J, Stroh T, Beaudet A. Potent spinal analgesia elicited through stimulation of NTS2 neurotensin receptors. J Neurosci. 2005;25:8188–8196. doi: 10.1523/JNEUROSCI.0810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrieau A, Javoy-Agid F, Kitabgi P, Dussaillant M, Vial M, Vincent JP, et al. Characterization and autoradiographic distribution of neurotensin binding sites in the human brain. Brain Res. 1985;348:375–380. doi: 10.1016/0006-8993(85)90461-5. [DOI] [PubMed] [Google Scholar]
- Schaeffer P, Laplace MC, Bernat A, Prabonnaud V, Gully D, Lespy L, et al. SR142948A is a potent antagonist of the cardiovascular effects of neurotensin. J Cardiovasc Pharmacol. 1998;31:545–550. doi: 10.1097/00005344-199804000-00012. [DOI] [PubMed] [Google Scholar]
- Schlaich MP, Lambert E, Kaye DM, Krozowski Z, Campbell DJ, Lambert G, et al. Sympathetic augmentation in hypertension: role of nerve firing, norepinephrine reuptake, and Angiotensin neuromodulation. Hypertension. 2004;43:169–175. doi: 10.1161/01.HYP.0000103160.35395.9E. [DOI] [PubMed] [Google Scholar]
- Shahid IZ, Rahman AA, Pilowsky PM. Intrathecal orexin A increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. Br J Pharmacol. 2011;162:961–973. doi: 10.1111/j.1476-5381.2010.01102.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Rybak IA, Paton JF. Structural and functional architecture of respiratory networks in the mammalian brainstem. Philos Trans R Soc Lond B Biol Sci. 2009;364:2577–2587. doi: 10.1098/rstb.2009.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spampinato S, Romualdi P, Candeletti S, Cavicchini E, Ferri S. Distinguishable effects of intrathecal dynorphins, somatostatin, neurotensin and s-calcitonin on nociception and motor function in the rat. Pain. 1988;35:95–104. doi: 10.1016/0304-3959(88)90281-3. [DOI] [PubMed] [Google Scholar]
- Spirovski D, Li Q, Pilowsky PM. Brainstem galanin synthesising neurons are differentially activated by chemoreceptor stimuli and represent a subpopulation of respiratory neurons. J Comp Neurol. 2011;520:154–173. doi: 10.1002/cne.22723. [DOI] [PubMed] [Google Scholar]
- Stanzione P, Zieglgansberger W. Action of neurotensin on spinal cord neurons in the rat. Brain Res. 1983;268:111–118. doi: 10.1016/0006-8993(83)90395-5. [DOI] [PubMed] [Google Scholar]
- St-Gelais F, Jomphe C, Trudeau LE. The role of neurotensin in central nervous system pathophysiology: what is the evidence? J Psychiatr Neurosci. 2006;31:229–245. [PMC free article] [PubMed] [Google Scholar]
- Stolakis V, Kalafatakis K, Botis J, Zarros A, Liapi C. The regulatory role of neurotensin on the hypothalamic-anterior pituitary axons: emphasis on the control of thyroid-related functions. Neuropeptides. 2010;44:1–7. doi: 10.1016/j.npep.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Takakura AC, Colombari E, Menani JV, Moreira TS. Ventrolateral medulla mechanisms involved in cardiorespiratory responses to central chemoreceptor activation in rats. Am J Physiol. 2011;300:R501–R510. doi: 10.1152/ajpregu.00220.2010. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Russell G, Spike RC. Immunocytochemical evidence that GABA and neurotensin exist in different neurons in laminae II and III of rat spinal dorsal horn. Neuroscience. 1992;47:685–691. doi: 10.1016/0306-4522(92)90176-3. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Spike RC, Price RF, Neilson M. Immunocytochemical evidence that neurotensin is present in glutamatergic neurons in the superficial dorsal horn of the rat. J Neurosci. 1994;14:774–784. doi: 10.1523/JNEUROSCI.14-02-00774.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler-McMahon BM, Boules M, Richelson E. Neurotensin: peptide for the next millennium. Regul Pept. 2000;93:125–136. doi: 10.1016/s0167-0115(00)00183-x. [DOI] [PubMed] [Google Scholar]
- Vincent JP. Neurotensin receptors: binding properties, transduction pathways, and structure. Cell Mol Neurobiol. 1995;15:501–512. doi: 10.1007/BF02071313. [DOI] [PubMed] [Google Scholar]
- Vincent JP, Mazella J, Kitabgi P. Neurotensin and neurotensin receptors. Trends Pharmacol Sci. 1999;20:302–309. doi: 10.1016/s0165-6147(99)01357-7. [DOI] [PubMed] [Google Scholar]
- Vita N, Oury-Donat F, Chalon P, Guillemot M, Kaghad M, Bachy A, et al. Neurotensin is an antagonist of the human neurotensin NT2 receptor expressed in Chinese hamster ovary cells. Eur J Pharmacol. 1998;360:265–272. doi: 10.1016/s0014-2999(98)00678-5. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Zogovic B, Pilowsky PM. Intrathecal bombesin is sympathoexcitatory and pressor in rat. Am J Physiol. 2011;301:R1486–R1494. doi: 10.1152/ajpregu.00297.2011. [DOI] [PubMed] [Google Scholar]


