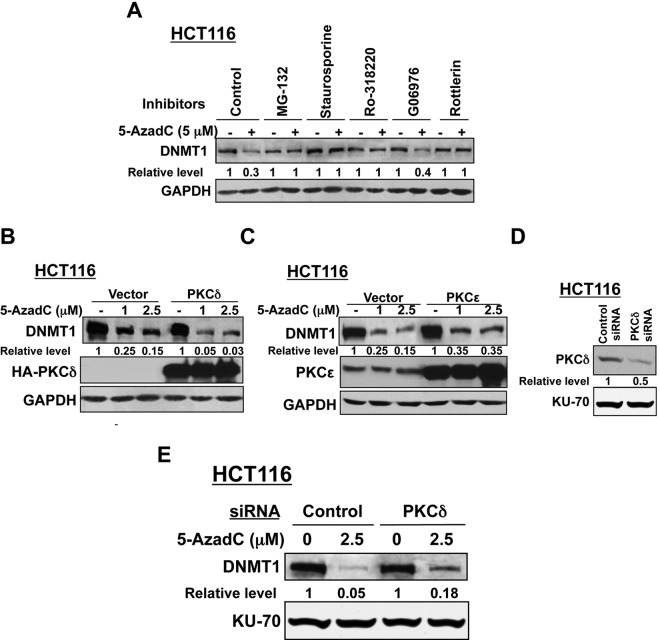
Figure 5.
(A) Decitabine-induced degradation of DNMT1 can be blocked by a PKCδ-specific inhibitor. Cells were treated with MG-132, staurosporine, broad-spectrum PKC inhibitor Ro-318220 (5 µM), PKCα or PKCδ inhibitors, G06976, or rottlerin (5-10 µM) for 30 minutes before treatment with decitabine (5 µM) for 8 hours. Whole cell extracts were subjected to Western blot analysis with anti-DNMT1 and anti-GAPDH antibodies. (B, C) Ectopic expression of the PKCδ protein facilitates decitabine-induced degradation of DNMT1, while PKCϵ exhibits a negligible effect. HCT116 cells were transfected with expression vectors (HA-tagged) of PKCδ wild-type (B), PKCϵ (C) wild-type, or corresponding empty vectors. Cells were equally distributed into 3 plates at 24 hours after transfection and 12 hours later were either left untreated or treated with decitabine (1 and 2.5 µM) for an additional 12 hours. Whole cell extracts (100 µg) were subjected to Western blot analysis with anti-DNMT1, anti-HA (for PKCδ expression), anti-PKCϵ, and anti-GAPDH antibodies. All quantitative results are the mean of 3 independent experiments. (D, E) Small interfering RNA (siRNA)–mediated depletion of PKCδ blocks decitabine-induced degradation of DNMT1. HCT116 cells were transfected with PKCδ siRNA or control siRNA. The cells were equally split into 2 at 24 hours following transfection and were either left untreated or treated with decitabine (2.5 µM) for an additional 12 hours. Western blot analysis (100 µg) was performed with specific antibodies. Representative figures are presented, and quantification results are the mean of 3 independent experiments.