Table 1.
Bioreduction of different substrates with SYE-4 proteins
| Entry | Substrate | Time | Conv. (%)a | ee (%) | Abs. config. | Product | |
|---|---|---|---|---|---|---|---|
| Ketones | |||||||
| 1 | 1a (R=2-Me) | 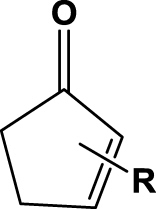 |
6 h | 92 | 52 | nd | 2a |
| 2 | 1b (R=3-Me) | 6 h | nc | na | na | 2b | |
| 3 | 1c | 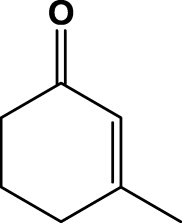 |
6 h | nc | na | na | 2c |
| 4 | 1d (n=1) | 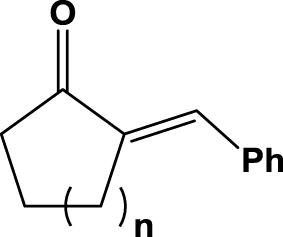 |
6 h | 4 | rac | na | 2d |
| 5 | 1e (n=2) | 6 h | 92 | 51 | nd | 2e | |
| 6 | 1f (n=1) | 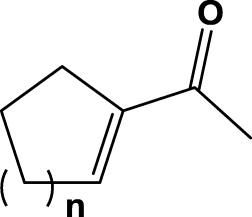 |
6 h | 30 | na | na | 2f |
| 7 | 1g (n=2) | 6 h | 71 | na | na | 2g | |
| 8 | 1h (+)-Carvone | 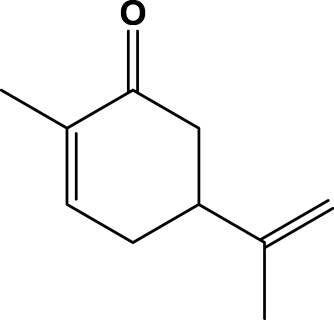 |
1 h | 100 | 97b | 2R,5R | 2h |
| 9 | 1i (−)-Carvone | 1 h | 100 | 95c | 2R,5S | 2i | |
| Diesters | |||||||
| 10 | 1j (R=Me) | 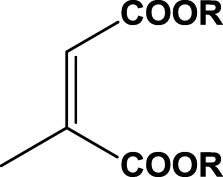 |
6 h | 99 | 98 | R | 2j |
| 11 | 1k (R=Et) | 6 h | 99 | 99 | R | 2k | |
| 12 | 1l (R=Me) | 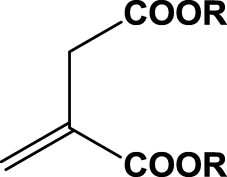 |
6 h | 19 | 90 | R | 2j |
| 13 | 1m (R=Et) | 6 h | nc | na | na | 2k | |
| Imides | |||||||
| 14 | 1n (R1=Me; R2=Me) | 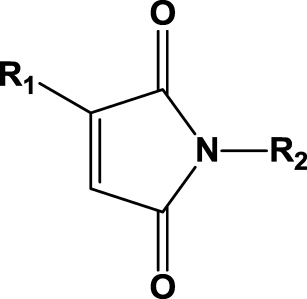 |
1 h | 99 | 99 | R | 2n |
| 15 | 1o (R1=Me; R2=Et) | 3 h | 99 | 99 | R | 2o | |
| 16 | 1p (R1=Me; R2=Propyl) | 3 h | >99 | 99 | R | 2p | |
| 17 | 1q (R1=Me; R2=Butyl) | 3 h | 99 | 99 | R | 2q | |
| 18 | 1r (R1=Me; R2=Allyl) | 3 h | 99 | 99 | R | 2r | |
| 19 | 1s (R1=Me; R2=Bn) | 3 h | 99 | 99 | R | 2s | |
| 20 | 1t (R1=H; R2=Allyl) | 1 h | 99 | na | na | 2t | |
| 21 | 1u (R1=H; R2=Bn) | 1 h | 98 | na | na | 2u | |
nd=not determined; na=not applicable.
Conversion based on GC.
Diastereomeric excess (trans).
Diastereomeric excess (cis).
