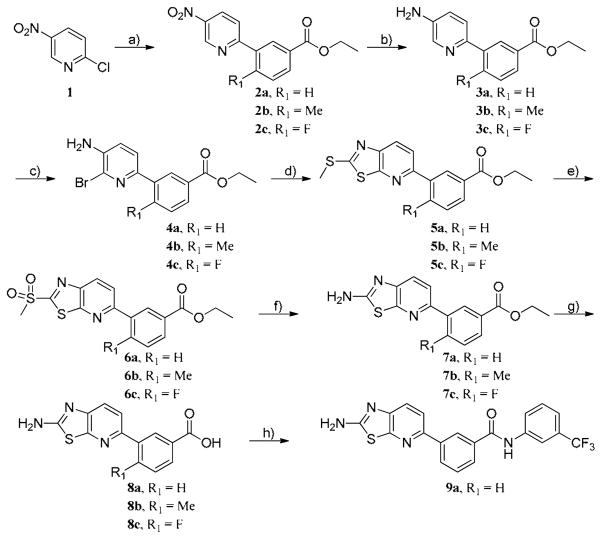
Scheme 1. Reagent and conditions.
a) Boronic acids[(3-(Ethoxycarbonyl)phenylboronic Acid(R1 = H), 5-Methoxycarbonyl-2-methylphenylboronic acid (R1 = Me), 5-Ethoxycarbonyl-2-fluorophenylboronic acid (R1 = F)], Pd(PPh3)2Cl2, tert-Butyl XPhos, 2N Na2CO3(aq), dioxane, 90 °C, 10 h, b) 5% Pd/C, EtOH, 16 h, c) NBS, DMF, 0 °C, 5-10 min, d) Potassium ethyl xanthogenate, AcOH, NMP, 150 °C, 16 h and MeI, 50 °C, 30 min, e) Oxone, MeOH, THF, Water, rt, 16 h, f) 2.0 N NH3 in IPA, 90 °C, 24 h, g) LiOH·H2O, THF, MeOH, H2O, rt, 16 h, h) HATU, DIEA, 3-(trifluoromethyl)aniline, DMF, rt, 16 h.