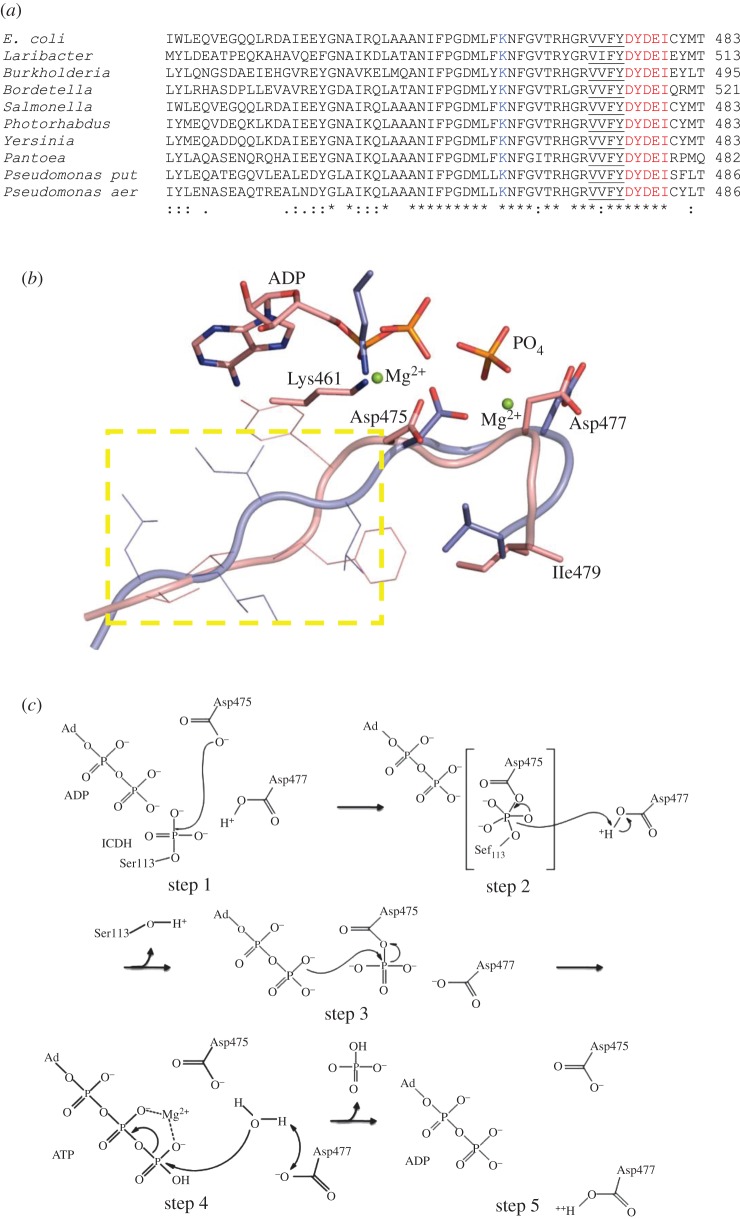
Figure 4.
(a) Sequence alignment of AceK from several species. The DXDX(T/V) motif is highlighted in red. The preceding four hydrophobic residues are underlined, and the conserved lysine involved in transition state stabilization is shown in blue. (b) Structural alignment of AceK 475DXDX(I)479 motif (pink) with that of d,d-heptose 1,7-bisphosphate phosphatase [31] (PDB accession code: 3L8H; light blue). The yellow rectangle contains the four hydrophobic residues, and the strictly conserved lysine coordinated with Mg2+ ion. (c) Proposed phosphatase mechanism for AceK. (step 1) Asp475 acts as the nucleophile attacking the phosphate moeity on Ser113 of IDH through an associative mechanism to form a pentacovalent phosphate intermediate. (step 2) Asp477 acts as a general acid to donate its proton to Ser113 of IDH. The phosphoenzyme intermediate at Asp475 is formed. (step 3) The aspartatyl-phosphate undergoes nucleophilic attack by the ‘catalytic residue’ ADP to produce ATP. The breaking of the high-energy apartatyl-phosphate bond provides the energy for this reaction. (step 4) Water donates its proton to Asp477 and then attacks the γ-phosphate of ATP (ATP hydrolysis reaction). (step 5) The completed reaction with the enzyme available to perform its next round of reaction.