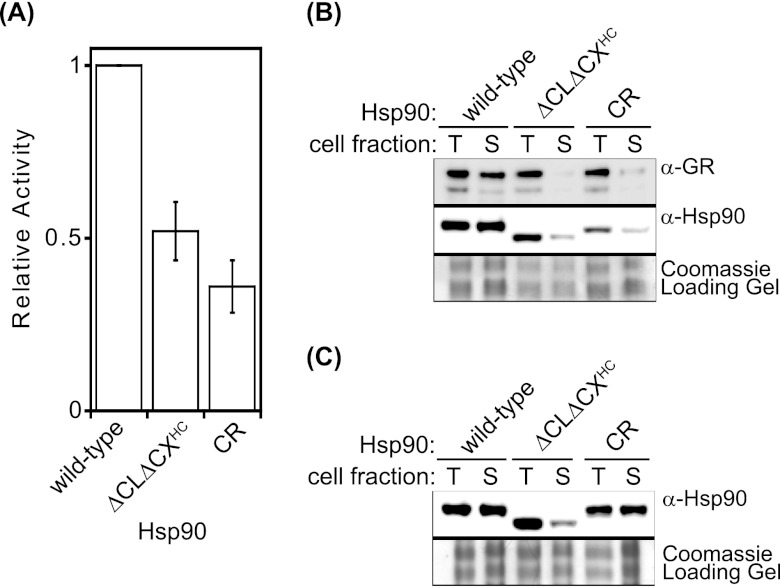
Fig 5.
ΔCLΔCXHC Hsp90 was unable to mature the hormone receptor GR in yeast. (A) GR reporter activity levels were lower than those of the wild type for both ΔCLΔCXHC and CR Hsp90. Error bars represent standard deviations (n = 3). (B) Western blotting for GR in whole-cell lysates (T) and soluble fractions (S) indicated that the ratio of soluble to total GR is drastically reduced in ΔCLΔCXHC and CR Hsp90 cells. The solubilities of ΔCLΔCXHC and CR Hsp90 were also reduced compared to that of wild-type Hsp90. (C) In the absence of exogenous client proteins, the solubility of ΔCLΔCXHC Hsp90 was drastically reduced, while CR Hsp90 maintained wild-type solubility.