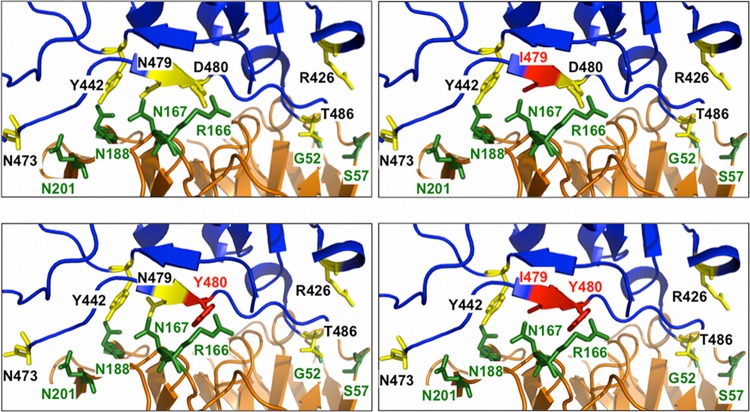
Fig 6.
Effect of escape mutations on SK4. (Top left) SK4 interacts robustly with RBD via polar interactions involving N167, N188, and N201 of SK4 with N473, Y442, and N479 of the RBD. An electrostatic interaction between D480 of the RBD and R166 of SK4 also contributes to robust binding. (Top right) An escape mutant with N479I would likely still interact strongly with SK4. While I479 would not participate in polar interactions with N167, N188, and N201, the electrostatic interaction between D480 of the RBD and R166 of SK4 would be sufficient to allow strong binding of SK4 to the RBD mutant. (Bottom left) A mutant with a D480Y change would knock out the electrostatic interaction between D480 of the RBD and R166 of SK4, but the polar interactions of N167, N188, and N201 of SK4 with N473, Y442, and N479 of the RBD would be sufficient to preserve strong binding between SK4 and this mutant. (Bottom right) Only the double mutation N479I plus D480Y would allow escape from SK4 binding, as this mutation would disrupt two robust binding residues. Residues 430 to 435 and 469 to 472 of the RBD have been removed to allow better visualization of the structures. Blue, RBD; orange, SK4; yellow, RBD-interacting residues; green, SK4-interacting residues; red, mutation sites 479I and 480Y.