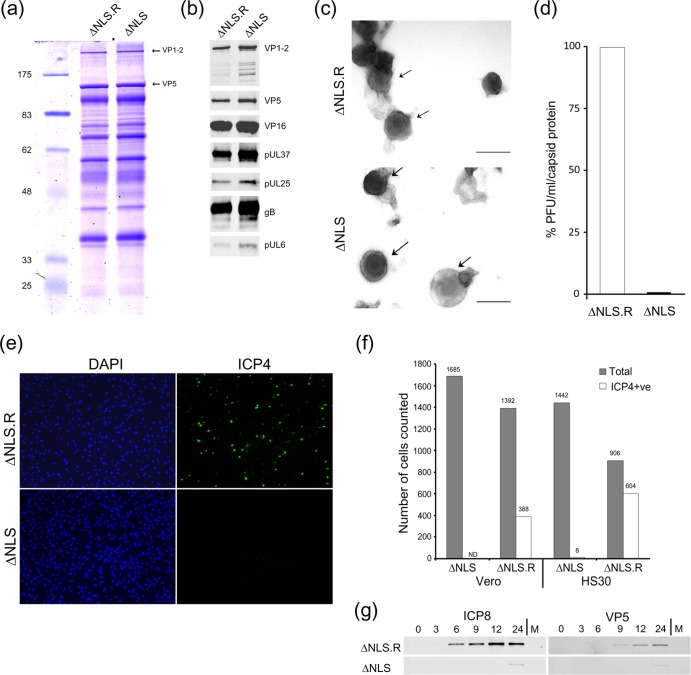
Fig 6.
Characterization of extracellular K.VP1-2ΔNLSNC virus produced from noncomplementing cells. HaCaT cells were infected with K.VP1-2ΔNLSC or the revertant virus at an MOI of 5 (estimated from titration on complementing cells). We used HaCaT cells because we find that they help to increase overall yields of all herpesvirus strains. The medium was harvested 16 h after infection and clarified, and extracellular virus was pelleted by centrifugation at 19,000 rpm for 90 min. Samples of pelleted virus showed slightly increased amounts of virion proteins for the mutant. Samples were adjusted (based on the major capsid protein VP5) and analyzed by SDS-PAGE. (a) Mutant and revertant viruses exhibited similar qualitative and quantitative profiles of structural proteins, including VP1-2 and VP5. (b) Equalized samples were analyzed by Western blotting for the presence of a number of structural components, as indicated. (c) Samples of extracellular particles from revertant- or mutant-infected cell medium, analyzed by negative staining. Various degrees of stain penetration were observed for both the mutant and the revertant, and examples illustrating partially stained intact particles are shown for both. Bars, 200 nm. (d) Comparison of specific infectivities of K.VP1-2ΔNLSNC and revertant extracellular virus particles, equalized on the basis of the major capsid protein and titrated for plaque formation on complementing HS30 cells. On a capsid-standardized basis, K.VP1-2ΔNLSNC particles exhibited approximately 600-fold lower infectivity. (e) Comparison of infectivities at the single-cell level. Vero or HS30 cells were infected with serial dilutions of the revertant virus designed to give low-MOI infections within the range of 1:3 to 1:100 cells infected. Cells were infected in parallel with K.VP1-2ΔNLSNC particles over an equivalent range based on equalized capsid protein. Cells were fixed 6 h after infection, stained with antibody to detect ICP4, and counterstained with DAPI (4′,6-diamidino-2-phenylindole) to enumerate cells. Numerous cells were counted for each virus at each dilution. Typical fields are shown for Vero cells with the largest amount of input virus to emphasize the difference in infectivity between the revertant and the mutant, as quantified in panel f. (g) Based on the titer of the revertant virus, cells were infected at a high MOI (5 PFU/cell) with the revertant virus or an equivalent input of mutant virus, based on standardization of the capsid protein in extracellular virus particles. Cells were harvested for Western blotting at the indicated times, with analysis in this case of a typical delayed early protein (ICP8) or the late major capsid protein VP5.