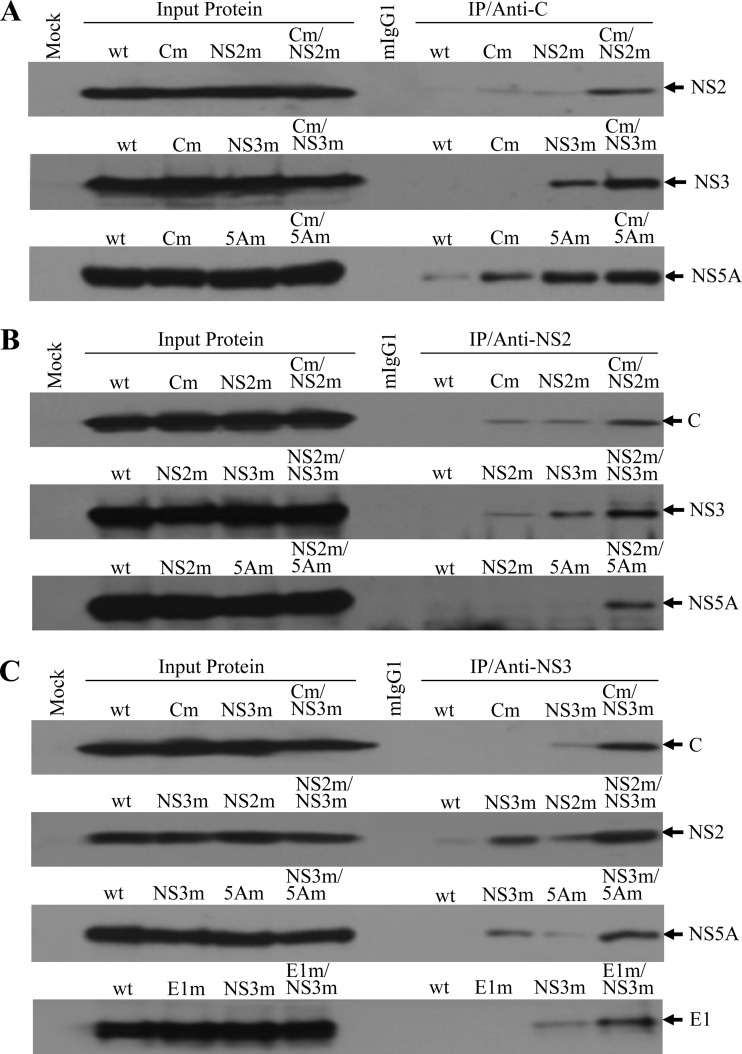
Fig 7.
HCV protein-protein interactions determined by co-IP. (A) Co-IP of HCV proteins using a C-specific monoclonal antibody. The wild-type and mutant JFH1 RNAs were transfected into Huh-7.5 cells as described in Materials and Methods. At 72 h p.t., the RNA-transfected cells were lysed in a RIPA buffer (50 mM Tris-HCl, pH 7.5, 150 mM sodium chloride, 1% Nonidet P-40, and 0.5% sodium deoxycholate). HCV proteins were coprecipitated using an HCV C-specific MAb (anti-C), which was bound to protein G-conjugated agarose beads. Upon co-IP, HCV NS2, NS3, and NS5A were detected by Western blotting using specific antibodies. (B) Co-IP of HCV proteins by an NS2 monoclonal antibody. The co-IP experiments were done in the same way as for panel A except that an NS2 MAb (anti-NS2) was used for IP. Upon co-IP, HCV C, NS3 and NS5A were detected by Western blotting. (C) Co-IP of HCV proteins by an NS3 monoclonal antibody. The co-IP experiments were carried out by incubating the cell lysates with an NS3-specific MAb (anti-NS3). Upon co-IP, HCV C, E1, NS2, and NS5A were detected by Western blotting using specific antibodies. The input lysate is shown on the left side, and IP with C-, NS2-, and NS3-specific monoclonal antibodies is on the right side. HCV proteins detected by Western blotting are highlighted on the right. “Mock” indicates Huh-7.5 cells without HCV proteins. A negative control for IP with a normal mouse IgG1 is indicated by mIgG1. The lower-case m stands for HCV proteins containing adaptive amino acid mutations.