Fig 5.
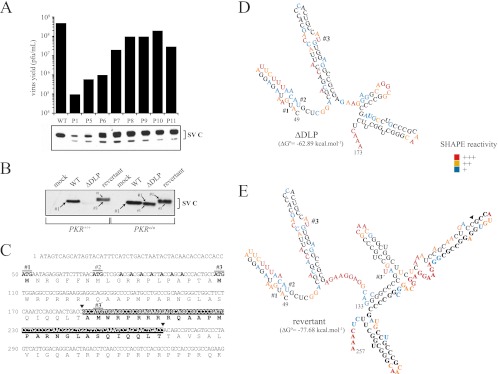
ΔDLP mutant virus evolved in murine cells to restore DLP activity. (A) Reversion from the 5th to 6th passages allowed fitness gain and rapid enrichment of cultures with revertant viruses. Viral yields for each passage were titrated. Capsid protein synthesis in NIH 3T3 cells infected with virus preparation from each passage is shown. (B) Revertant virus restored translation of 26S mRNA in NIH 3T3 cells. WT and PKRo/o cells were infected with the reconstituted revertant virus, and the synthesis of capsid protein was compared with that of the WT and parental ΔDLP mutant. Capsid bands were numbered according to the AUG codon from which they are translated. The sizes of the arrows are proportional to the translational efficiency from each AUG. (C) Genotype of revertant virus. The first 350 nt of 26S mRNA are shown. Arrowheads mark the insertion of the 84-nt fragment that resulted from the tandem duplication of the RNA fragment (see the text for details). AUG 1 (#1) is the natural initiation codon AUG50, AUG 2 (#2) is AUG72, and AUG 3 (#3) is AUG107. Note that the revertant has a duplicated no. 3 AUG (termed 3′), which was used as an initiation codon at an extremely low rate. The seven A's labeled in boldface are the mutations introduced in the parental WT virus to destroy the DLP structure. (D) RNA secondary structure of ΔDLP virus. MFE corresponds to nt 49 to 257 of 26S mRNA, although only the structure for the fragment from nt 49 to 173 is shown. Shape reactivity was scored as follows: +++, 50 to 100% of maximal; ++, 25 to 50% of maximal; +, <25% of maximal. Noncolored bases show no reactivity. (E) RNA secondary structure of 49 to 257 nt of revertant virus 26S mRNA. Note the new stem-loop formed at 26 nt downstream of AUG 3. Nucleotides that resulted from the tandem duplication are in bold, and the arrowhead indicates the site of insertion. Shape reactivity was scored as indicated above.