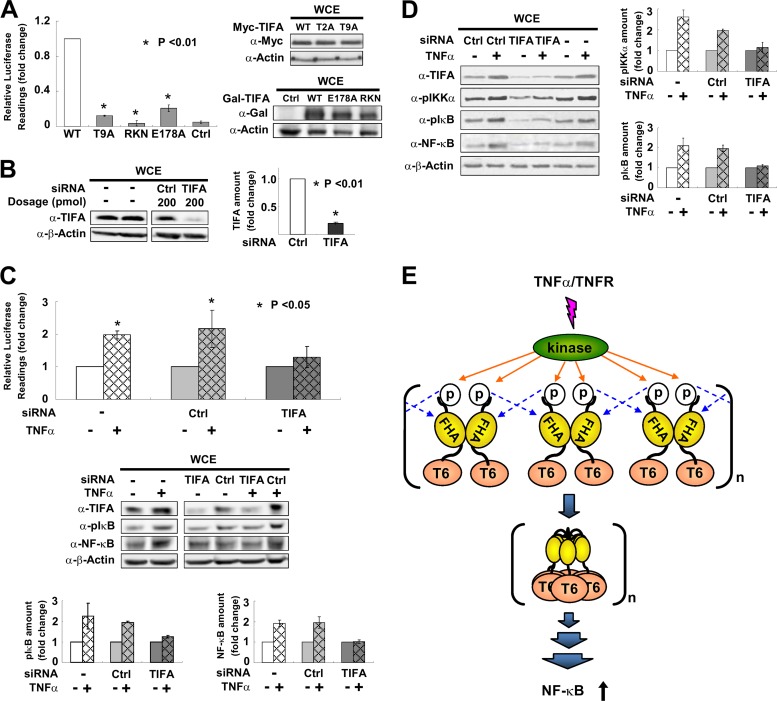
Fig 5.
pT9-FHA binding is involved in TNF-α-mediated activation of NF-κB. (A) HEK 293T cells were transfected with an NF-κB luciferase reporter together with WT TIFA or its various mutants. NF-κB activation was evaluated by luciferase activity assays. The bar graph represents fold changes of normalized relative luminescence units (RLU). The results are mean ± SD from at least 3 independent experiments. Western blotting on the right shows the expression profiles of mutants used in the experiments. (B) HEK 293T cells were transfected with TIFA siRNA or scramble RNA (Ctrl). The efficiency of RNA interference was verified by Western blotting in at least 3 independent experiments and plotted in the bar graph shown on the right. (C) HEK 293T cells receiving TIFA siRNA or scramble RNA (Ctrl) were cotransfected with an NF-κB luciferase reporter and then stimulated with TNF-α as indicated. NF-κB activation was evaluated by luciferase activity assays. The values for the untreated groups were set as 1. The cell lysates were also analyzed by Western blotting. The bar graphs at the bottom represent the fold changes of phosphorylated IκB (pS32/pS36) and total NF-κB (p65) proteins after TNF-α treatment. (D) The experimental conditions for siRNA transfection were the same as those described for panel C except that the cell line used was THP-1 (a human acute monocytic leukemia cell line). The bar graphs represent the fold changes of phosphorylated IKKα (pT23) and phosphorylated IκB (pS32/pS36) after TNF-α stimulation. (E) Proposed model for TIFA oligomerization, which first takes place via intermolecular FHA-pT9 binding between TIFA dimers upon TNF-α stimulation and then leads to TRAF6 (labeled T6) oligomerization and subsequent NF-κB activation.