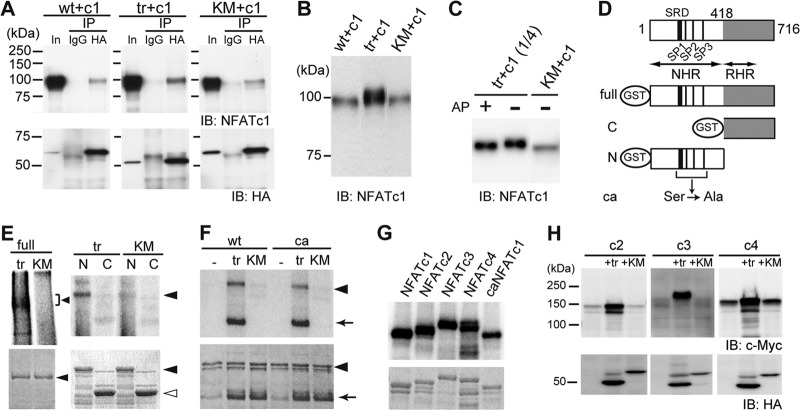
Fig 3.
Cot binds to and directly phosphorylates NFATc1. (A) Interaction of wild-type (wt), active (tr), and kinase-deficient (KM) forms of HA-tagged Cot with NFATc1 (c1) in HeLa cells by immunoprecipitation (IP) assay. In, input; IB, immunoblotting. (B) Increased expression and slower migration of NFATc1 in HeLa cells cotransfected with trCot, as revealed by comparison with the input lanes in panel A (detected by short exposure time). (C) NFATc1 is hyperphosphorylated in HeLa cells coexpressing trCot but not in cells expressing KMCot. Alkaline phosphatase (AP) treatment increased mobility of NFATc1, presumably through removal of Cot-induced phosphate groups. (D) Schematic representation of the structure of various GST fusion fragments of NFATc1. SRD, serine-rich domain. SP1, SP2, and SP3 are highly conserved Ser-Pro (SP) repeat motifs reportedly responsible for nuclear localization. NHR, NFAT homology region; RHR, Rel homology region; ca, constitutively active form. (E) Cot directly phosphorylates NFATc1. (Top) Cot phosphorylates the full-length (left, arrowhead) and the N-terminal region (right, arrowhead), but not the C-terminal region of NFATc1, as detected by autoradiography. (Bottom) Coomassie brilliant blue (CBB) staining. Closed arrowhead, full-length (left) or N-terminal region (right) of NFATc1. Open arrowhead, C-terminal region of NFATc1. (F) The N-terminal region of a constitutively active form (ca) of NFATc1 is also phosphorylated by Cot. Arrow, autophosphorylated trCot. (G) Cot directly phosphorylates the N-terminal region of Ca2+/calcineurin-regulated NFAT family proteins. (Top) trCot phosphorylates the N-terminal regions of NFATc2, NFATc3, and NFATc4 as detected by autoradiography. (Bottom) Coomassie brilliant blue (CBB) staining. (H) Increased protein levels of indicated Ca2+/calcineurin-regulated NFAT family proteins in HeLa cells cotransfected with trCot.