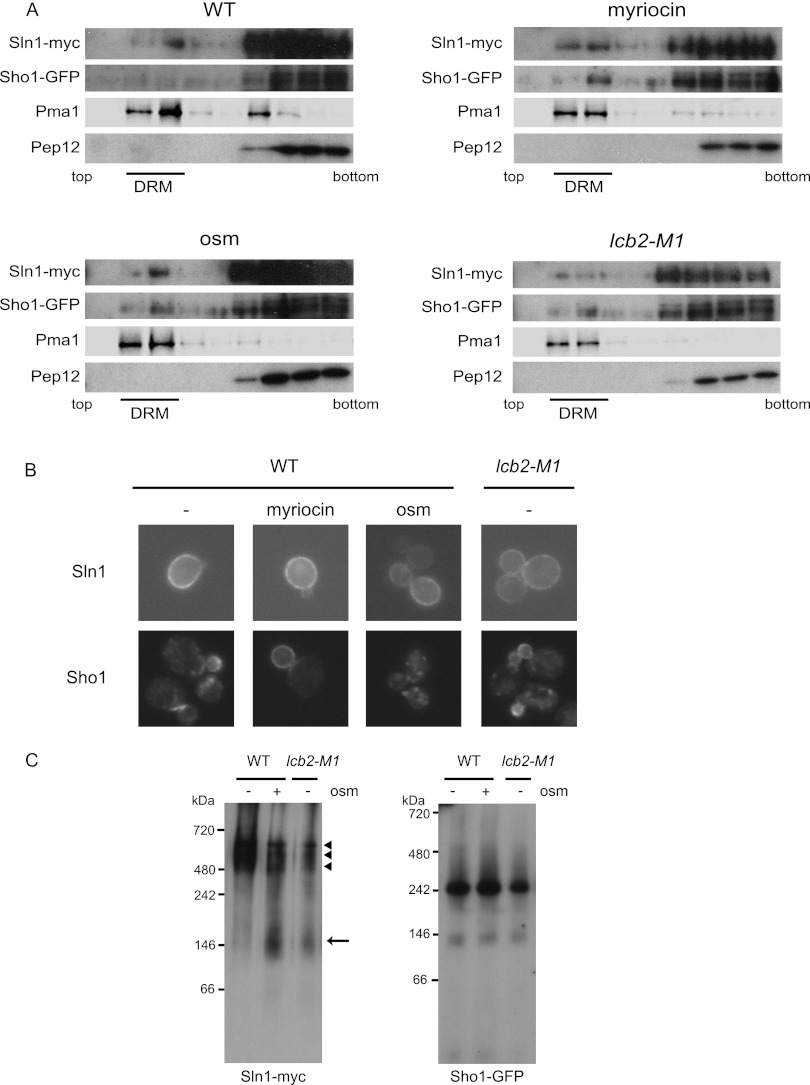
Fig 6.
DRM localization and complex formation of Sln1 and Sho1. (A) Sln1 and Sho1 localize in DRMs. SLN1-9myc SHO1-GFP (MH335) cells that were treated with 0.4 M KCl for 5 min (osm) or with 2 μg/ml myriocin for 2.5 h (myriocin) and untreated wild-type (MH335, WT) or lcb2-M1 SLN1-9myc SHO1-GFP (MH337, lcb2-M1) cells were disrupted, and their membrane fractions were extracted with 20 mM CHAPS and subjected to Optiprep density gradient centrifugation. Nine fractions were collected from the top and were analyzed by Western blotting using anti-myc, -GFP, -Pma1, and -Pep12 antibodies. Pma1 was used as a marker for DRM-associated proteins and Pep12 as a marker for non-DRM-associated membrane proteins. Fractions 2 and 3 contain DRMs. (B) Cellular localization of Sln1 and Sho1 in sphingolipid synthesis-deficient cells. Wild-type (TM141, WT) and lcb2-M1 (MH280) cells harboring an SLN1-GFP- or an SHO1-GFP-encoding plasmid were grown in SC-Ura. Wild-type cells were treated or not treated (−) with 2 μg/ml myriocin for 2.5 h (myriocin) or 0.4 M KCl for 5 min (osm). (C) Detection of Sln1 and Sho1 complexes by BN-PAGE. The membranes of SLN1-9myc SHO1-GFP (MH335, WT) cells and lcb2-M1 SLN1-9myc SHO1-GFP (MH337, lcb2-M1) cells that had been treated (osm +) or not treated (osm −) with 0.4 M KCl for 5 min were isolated, solubilized with 1% digitonin, and then subjected to BN-PAGE. After electrophoresis, Western blotting was performed using anti-myc and anti-GFP antibodies. An arrow and arrowheads indicate the fast- and the slow-migrating species of Sln1, respectively.