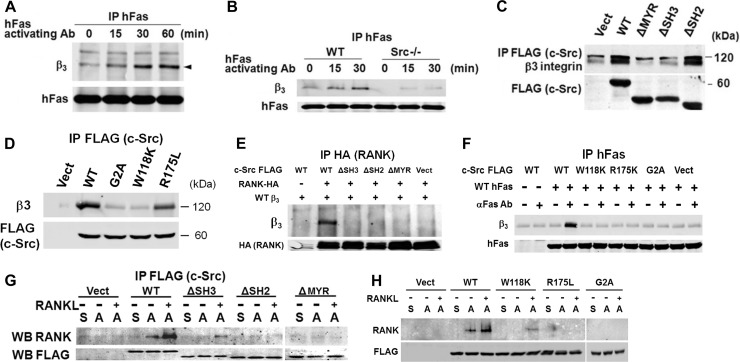
Fig 7.
c-Src links RANK and αvβ3. (A) WT preosteoclasts, transduced with WT hFas/RANK, were exposed to hFas MAb for the times shown. hFas immunoprecipitates (IP) were immunoblotted for β3 subunit and hFas. (B) WT and c-Src−/− preosteoclasts, transduced with WT hFas/RANK, were exposed to hFas MAb with time. hFas immunoprecipitates were immunoblotted for the β3 subunit and hFas. (C) Lysates of c-Src−/− preosteoclasts transduced with FLAG-tagged c-Src domain deletion constructs were immunoprecipitated with anti-FLAG MAb, and the product was immunoblotted for β3 subunit and FLAG. Vect, vector. (D) Lysates of c-Src−/− preosteoclasts transduced with FLAG-tagged c-Src point mutants were immunoprecipitated with anti-FLAG MAb, and the product was immunoblotted for the β3 subunit and FLAG. (E) HEK293T cells were transfected with FLAG-tagged c-Src constructs, HA-tagged RANK, and hβ3. HA immunoprecipitates were immunoblotted for hβ3 and HA. (F) Cytokine-starved c-Src−/− preosteoclasts transduced with FLAG-tagged c-Src point mutants and WT hFas/RANK were exposed to hFas MAb for 20 min. hFas immunoprecipitates were immunoblotted for β3 subunit and hFas. (G) Cytokine-starved, c-Src−/− prefusion osteoclasts, transduced with FLAG-tagged c-Src domain-deleted constructs, were maintained in suspension (S) or plated on vitronectin (A) for 30 min ± RANKL. FLAG immunoprecipitates were immunoblotted for RANK and FLAG. WB, Western blot. (H) Cytokine-starved, c-Src−/− prefusion osteoclasts, transduced with FLAG-tagged c-Src point mutants, were maintained in suspension (S) or plated on vitronectin (A) for 30 min ± RANKL. FLAG immunoprecipitates were immunoblotted for RANK and FLAG. The experiments were repeated 2 or 3 times with similar results.