Fig 2.
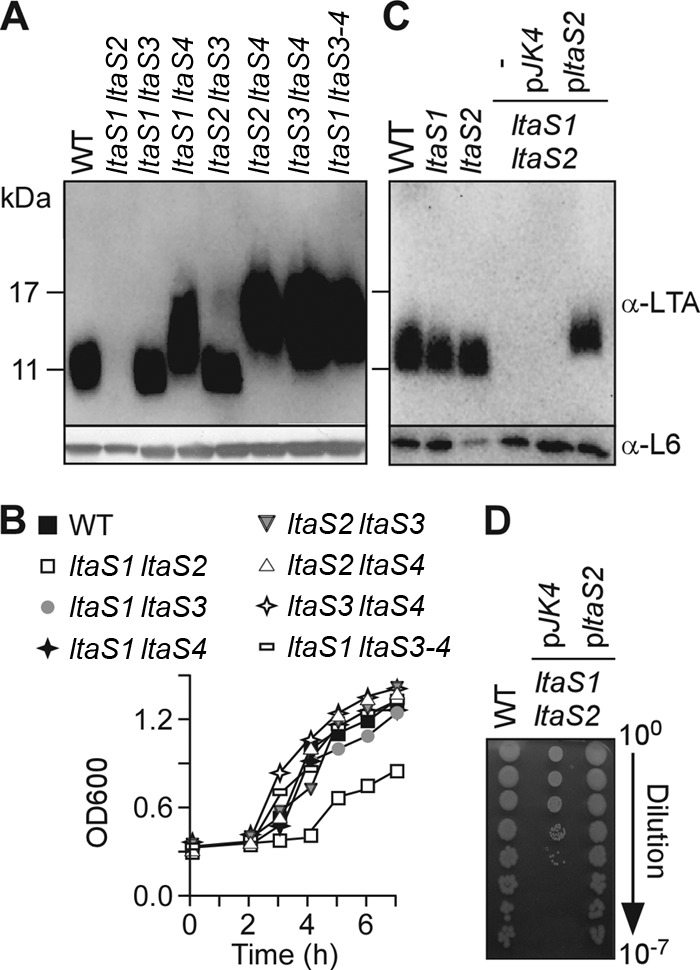
B. anthracis ltaS1 and ltaS2 are required for LTA synthesis and bacterial growth. (A) Immunoblot analyses of bacterial extracts isolated from the WT, the ltaS1 ltaS2, ltaS1 ltaS3, ltaS1 ltaS4, ltaS2 ltaS3, ltaS2 ltaS4, and ltaS3 ltaS4 double mutants, and the ltaS1 ltaS3 ltaS4 triple mutant by using antibodies specific for LTA, and ribosomal protein L6 (L6, loading control). The migration of protein size standards on SDS/PAGE gels is indicated in kilodaltons. (B) Growth curves of bacterial strains shown in panel A. Bacteria were diluted in BHI and grown at 37°C with shaking. Bacterial growth was monitored by optical density measurements of cultures at 600 nm (OD600). (C) Expression of ltaS2 on a plasmid restores LTA synthesis in the ltaS1 ltaS2 double mutant. Immunoblot analyses were performed as for panel A to compare LTA production among the WT, the ltaS1 or ltaS2 mutant, and the ltaS1 ltaS2 double mutant carrying no plasmid (minus), the plasmid with no insert (pJK4), or the plasmid carrying ltaS2 (pltaS2). (D) Expression of ltaS2 on a plasmid restores growth of the ltaS1 ltaS2 mutant. Bacterial growth was examined by plating serial dilutions (0- to 7-fold) for the WT, ltaS1 ltaS2 pJK4, and ltaS1 ltaS2 pltaS2.
