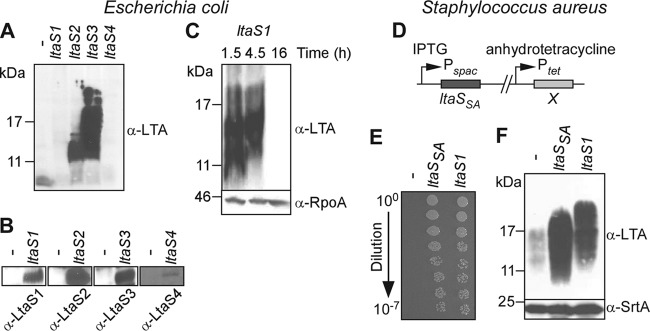
Fig 3.
B. anthracis LtaS1, LtaS2, and LtaS3 catalyze LTA synthesis in heterologous hosts. (A to C) Expression of B. anthracis LtaS1, LtaS2, and LtaS3 in E. coli promotes synthesis of polyglycerol phosphate. E. coli strains carrying pJK4 with no insert (minus) or with an insert (ltaS1, ltaS2, ltaS3, and ltaS4) were grown for 16 h in the presence of IPTG (A and B), and E. coli pJK4-ltaS1 was grown for 1.5, 4.5, and 16 h in the absence of IPTG (C). Extracts from normalized culture aliquots were separated by SDS-PAGE for immunoblot detection of LTA (A to C), LtaS enzymes, and RpoA (loading control) using specific antibodies (B). (D to F) Functional complementation of ltaS depletion in S. aureus with B. anthracis ltaS1 homologue. (D) Schematic representation of chromosomal organization in ltaS complementation strains. S. aureus cultures of strains carrying either no insert (minus), S. aureus ltaSSA, or B. anthracis ltaS1 at position X were grown overnight in the presence of IPTG. The following day, cultures were washed and diluted in fresh medium containing anhydrotetracycline only and analyzed for restoration of staphylococcal growth (E) as described for Fig. 2D and LTA production (F) by using antibodies specific for LTA and sortase A (SrtA; loading control). The values to the left of panels A, C, and F indicate the migration of protein size standards, in kilodaltons.