Fig 3.
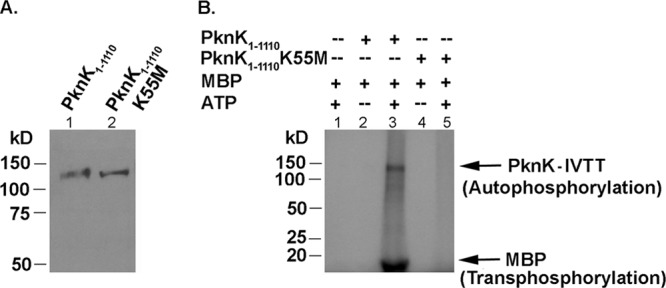
PknK proteins synthesized in a cell-free expression system are catalytically active. (A) IVTT-synthesized His-tagged PknK1-1110 (lane 1) and PknK1-1110K55M (lane 2) were resolved on a 10% SDS-PAGE gel and confirmed by Western blot analysis using the anti-His antibody. Recombinant PknK proteins of ∼130-kDa molecular mass were detected. (B) Kinase activity of IVTT-synthesized PknK proteins. In vitro kinase assays were carried out to test the auto- and transphosphorylation abilities of the wild-type or mutant PknK1-1110 kinase using MBP as a universal substrate protein in reactions with or without [γ-32P]ATP, followed by resolution on a 4 to 15% gradient SDS-PAGE gel. Radioactive signals were detected by phosphorimaging. The arrows indicate the bands corresponding to autophosphorylated PknK1-1110 and transphosphorylated MBP.
