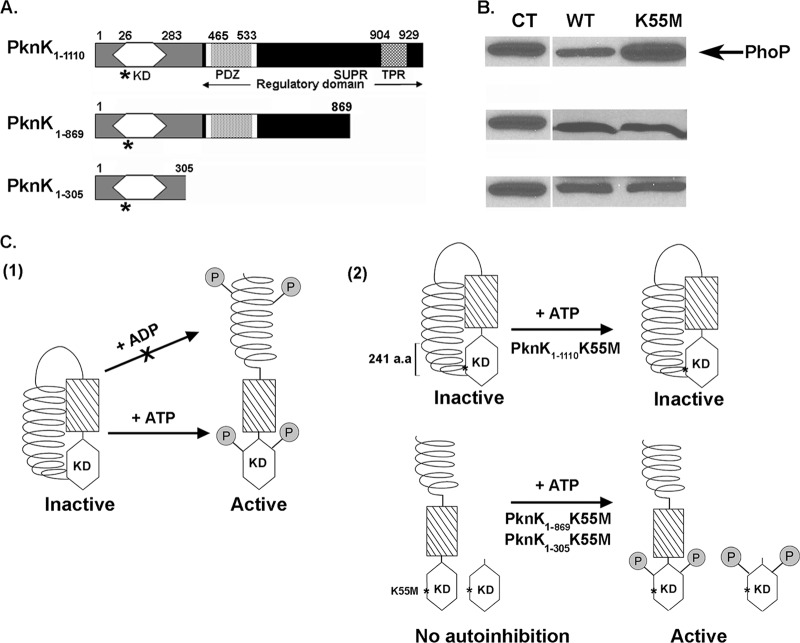
Fig 7.
Model for PknK activation and autoregulation: role of the C-terminal regulatory region. (A) Schematic linear representation of the various proposed domains in the full-length and truncated PknK proteins: a kinase domain (amino acids 26 to 283), an AAA motif (white box) encompassing the ATP/GTP-binding site (amino acids 368 to 375), a PDZ domain (amino acids 465 to 533), a helical region containing SUPR repeats (amino acids 777 to 1108), and a putative TPR-like motif (amino acids 904 to 929) (1, 27, 28; http://www.ebi.ac.uk/tools/pfa/iprscan and http://myhits.isb-sib.ch/cgi-bin/motif_scan). Truncated PknK proteins were constructed so that the PknK1-305 protein lacked the entire C-terminal regulatory region while the PknK1-869 lacked only the region containing the TPR-like helical motif (Δ241 amino acids). The K55M mutations in the proteins are marked with asterisks. (B) The PknK wild-type (WT) and mutant (K55M) proteins of various lengths were assayed for their inhibitory effects on IVTT of the target protein (PhoP) in the presence of ATP. CT, positive control for PhoP synthesis in the presence of pYA1635 (phoP) DNA alone. (C) Proposed model for PknK activation and autoregulation. For the sake of clarity, PknK is shown as a monomer; however, we have demonstrated that it can exist as oligomers of different sizes in solution. (1) Based on the results presented in this study, a hypothetical model for PknK activation (in terms of its ability to inhibit the IVTT reaction) is presented wherein, in the absence of any inducer, the interactions between the PknK C-terminal region and the N-terminal active site result in an inactive or autoinhibited kinase. Addition of a positive activator, such as ATP, results in conformational changes that relieve the autoinhibition and direct the autophosphorylation and activation of PknK. Similar structural changes resulting in PknK activation do not occur in the presence of ADP (arrow with an X). (2) Autophosphorylation of threonine residues in the PknK activation loop and some yet-unknown residues in the C-terminal region has been reported (27) and is depicted arbitrarily. The K55M mutation in the full-length protein excludes any possibility of ATP binding and hydrolysis and thus does not respond to structural activation by ATP, resulting in an inactive enzyme. Deletion of the C-terminal region in the truncated PknK1-869- and PknK1-305K55M proteins removes the autoregulatory control and allows ATP binding, resulting in an active protein. a.a, amino acids.