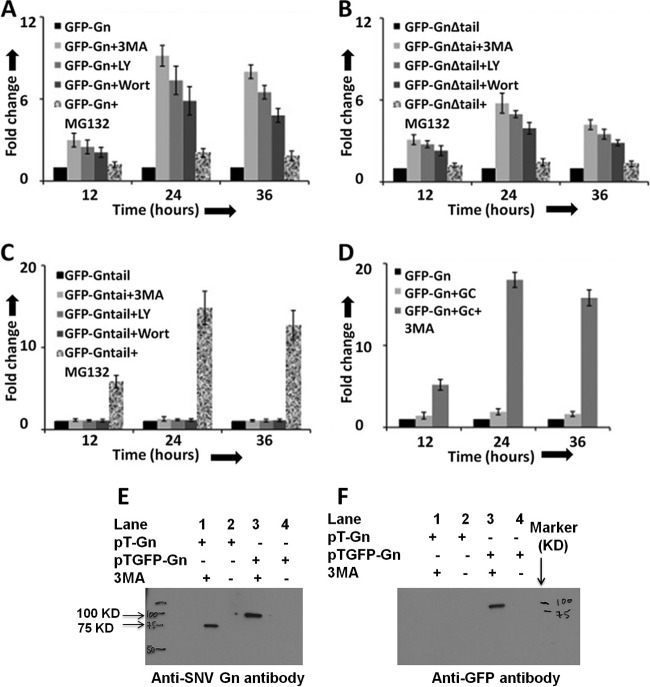
Fig 4.
Autophagy inhibitors protect the degradation of Gn in cells. HeLa cells in six-well plates were transfected with 2 μg of either pTGFP-Gn (A) or pTGFP-GnΔtail (B) or pTGFP-Gntail (C) construct, expressing GFP fused to either wild-type Gn (GFP-Gn), a mutant Gn devoid of the tail domain (GFP-GnΔtail), or the tail domain of Gn (GFP-Gntail), respectively. The medium containing transfection mixture was aspirated at 5 h posttransfection and replaced with fresh medium containing either 3-MA (10 mM final concentration), LY-294002 (LY; 50 μM final concentration), Wortmanin (Wort; 100 μM final concentration), MG132 (10 μM final concentration), or medium lacking the inhibitor (black bars). In each well a total of 4 μg of plasmid DNA was transfected, containing 2 μg of expression plasmid and 2 μg of the empty vector pT1.1. (D) Cells in six-well plates were cotransfected with 2 μg each of pTGFP-Gn and empty vector pT1.1 (black bars), pTGFP-Gn and pTGC plasmids lacking 3-MA (light gray bars), or pTGFP-Gn and pTGC plasmids containing 10 mM 3-MA (dark gray bars). The plasmid pTGC expresses untagged Gc. Fold change in panels A to D was calculated by normalizing the GFP signal to levels represented by the dark bar in the corresponding panel. (E) A Western blot showing Gn and GFP-Gn fusion protein using anti-Gn antibody. (F) Samples from panel E were examined by Western blot analysis using anti-GFP antibody. Western blot analysis (panels E and F) was carried out as described in Materials and Methods.