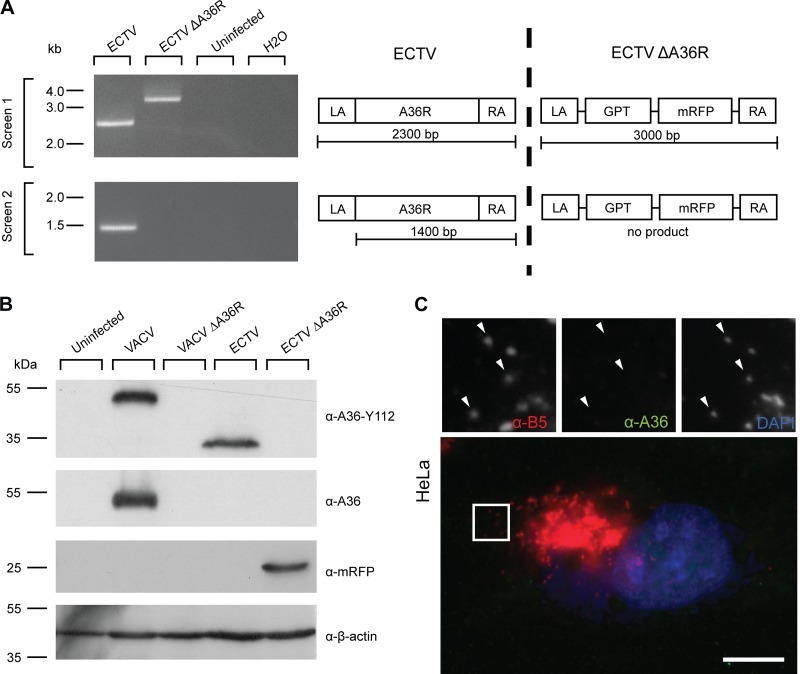
Fig 4.
Construction and verification of ECTV ΔA36R. (A) BSC-1 cells were infected with ECTV or recombinant ECTV ΔA36R, and cell lysates were collected at 48 hpi. Genomic DNA was extracted and PCR conducted with two primer pairs, a36r.lafor-a36r.rarev and a36r.for-a36r.raev, to confirm deletion of A36R and insertion of the selection cassette. Uninfected cell lysate genomic DNA and a no-template control were run simultaneously as negative controls. Molecular size markers are indicated on the left. A schematic of the PCRs performed is represented on the right. (B) BSC-1 cells were infected with VACV, VACV ΔA36R, ECTV, or ECTV ΔA36R at an MOI of 1, and cell lysates were collected at 16 hpi (VACV) or 48 hpi (ECTV). Cell lysates were separated by SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting with anti-A36 (raised against the C terminus of VACV A36, which is absent in ECTV A36), anti-A36-Y112, anti-mRFP, and anti-β-actin (as a loading control). Molecular size markers are indicated on the left. (C) HeLa cells were infected with ECTV ΔA36R, fixed, and stained for immunofluorescence assays with anti-B5 (NPC) (red), anti-A36-Y112 (green), and DAPI (blue). Arrowheads indicate WV. Bar, 10 μm.