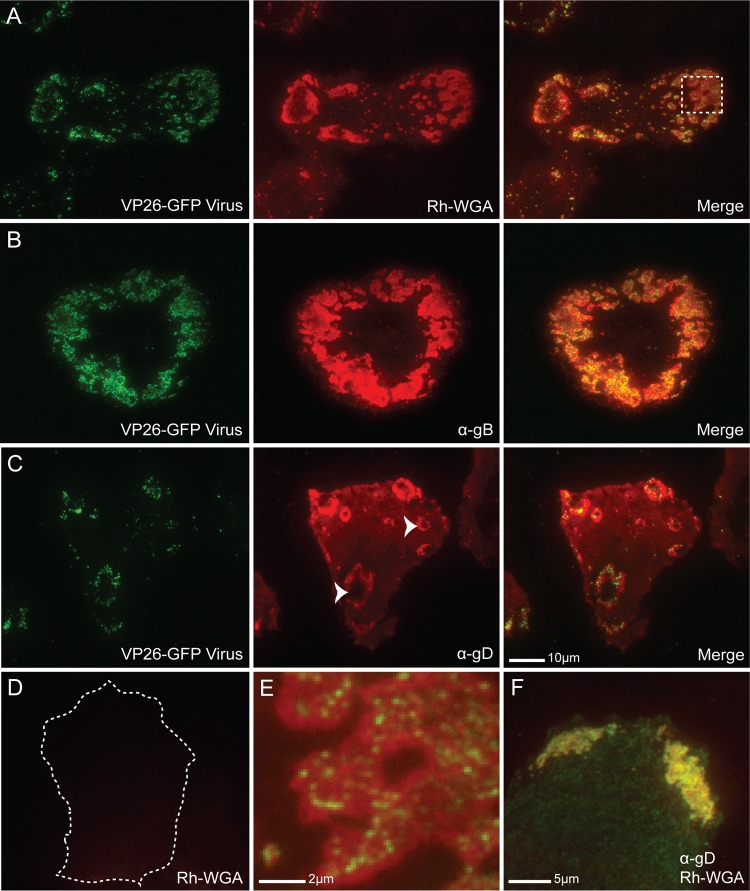
Fig 2.
Glycoprotein enrichment at coverslip-adherent surface egress sites. (A) TIRF micrographs of a VP26-GFP HSV-1-infected Vero cell at 12 hpi fixed, permeabilized, and stained with rhodamine-conjugated WGA (Rh-WGA) to mark glycosylated proteins. As in EM pictures, GFP-labeled virions were found to cluster at specific sites along the cell surface. Note that glycosylated proteins accumulate at these sites. A magnified image of the box is shown in panel E. (B) TIRF micrographs of a VP26-GFP HSV-infected Vero cell at 12 hpi that had been treated with α-gB (DL16) antibody. (C) TIRF micrographs of a VP26-GFP HSV-infected Vero cell treated with α-gD antibody (DL11). Staining indicates that viral glycoproteins accumulate at egress sites. Arrowheads mark two egress sites where the pocket-like structure of the sites is apparent. The holes in the donuts are areas where the membrane has extended beyond the 300-nm laser excitation range. Virus is rarely visible in the holes, indicating that virions are closely bound to the cell membrane. (D) Mock-infected Vero cell permeabilized and stained with WGA shown at that same exposure as that for the infected cell in panel A. The cell edge is outlined as determined by increasing the brightness of the image until the edge was visible. The size bar in panel C is also relevant for panels A to D. (F) HSV-1-infected Vero cell stained with both rhodamine-WGA and α-gD antibody. Note that there is complete colocalization between the two. Alexa 594-conjugated secondary antibodies were used in panels B, C, and F.