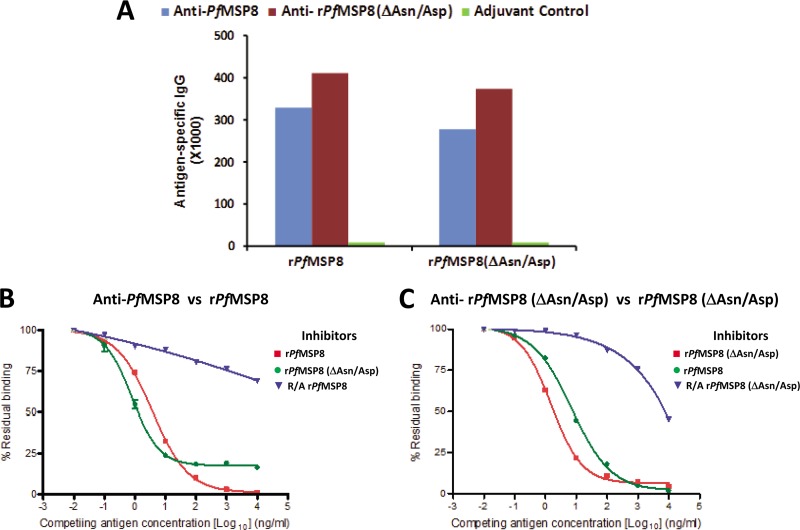
Fig 2.
Deletion of the Asn/Asp-rich domain does not alter the conformational integrity or B cell immunogenicity of rPfMSP8. (A) Antigen-specific IgG titers in sera of rabbits immunized with rPfMSP8 (blue), rPfMSP8(ΔAsn/Asp) (red), or adjuvants alone (green) were compared by direct-binding ELISA on plates coated with homologous or heterologous antigens as indicated on the x axis. For competitive ELISAs, a constant concentration of 5 ng/ml of anti-rPfMSP8 (B) or anti-rPfMSP8(ΔAsn/Asp) (C) IgG was preincubated with increasing concentrations of intact homologous antigen (■), intact heterologous antigen (●), or R/A homologous antigen (▼), and residual binding to plate-bound homologous antigen was determined by ELISA. The percent residual binding at various competing antigen (inhibitor) concentrations was calculated as (OD of IgG in the presence of inhibitor/OD of same IgG without inhibitor) × 100.