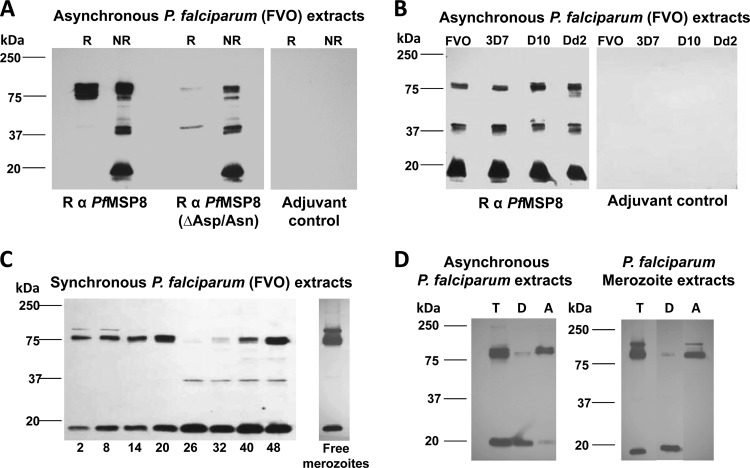
Fig 5.
Native PfMSP8 undergoes proteolytic processing yielding a 17-kDa, C-terminal membrane-anchored product. (A) Lysates of asynchronous P. falciparum (FVO) parasites were separated by SDS-PAGE under reducing (R) and nonreducing (NR) conditions and subjected to immunoblot analysis using rabbit anti-rPfMSP8 or anti-rPfMSP8(ΔAsn/Asp) IgG, with IgG obtained from rabbits immunized with adjuvant alone serving as a negative control. (B) Lysates of asynchronous P. falciparum 3D7, D10, and Dd2 parasites were subjected to immunoblot analysis under nonreducing conditions and probed with rabbit anti-rPfMSP8 IgG or adjuvant control IgG. (C) Lysates of equal numbers of stage-separated, synchronous, P. falciparum (FVO) parasites were subjected to immunoblot analysis under nonreducing conditions and probed with rabbit anti-rPfMSP8 IgG. (D) Asynchronous parasite or free merozoite pellets were lysed in the presence of ice-cold Triton X-114 and sampled for total antigen (T) before being subjected to temperature-dependent phase separation. The resulting detergent and aqueous phases were subjected to a second cycle of phase separation to yield the final detergent phase (D) and Triton X-114 depleted aqueous phase (A). Following SDS-PAGE under nonreducing conditions, aliquots of each sample were subjected to immunoblot analysis with rabbit anti-PfMSP8 IgG. All IgGs (A to D) were used at a final concentration of 1 μg/ml. In each panel, molecular weight markers in kilodaltons (kDa) are indicated.