Abstract
Invasion of intestinal epithelial cells by Salmonella enterica serovar Typhimurium is an energetically demanding process, involving the transfer of effector proteins from invading bacteria into host cells via a specialized organelle known as the Salmonella pathogenicity island 1 (SPI-1) type 3 secretion system (T3SS). By a mechanism that remains poorly understood, entry of S. Typhimurium into epithelial cells is inhibited by Sal4, a monoclonal, polymeric IgA antibody that binds an immunodominant epitope within the O-antigen (O-Ag) component of lipopolysaccharide. In this study, we investigated how the binding of Sal4 to the surface of S. Typhimurium influences T3SS activity, bacterial energetics, and outer membrane integrity. We found that Sal4 treatment impaired T3SS-mediated translocon formation and attenuated the delivery of tagged effector proteins into epithelial cells. Sal4 treatment coincided with a partial reduction in membrane energetics and intracellular ATP levels, possibly explaining the impairment in T3SS activity. Sal4's effects on bacterial secretion and energetics occurred concurrently with an increase in O-Ag levels in culture supernatants, alterations in outer membrane permeability, and changes in surface ultrastructure, as revealed by transmission electron microscopy and cryo-electron microscopy. We propose that Sal4, by virtue of its ability to bind and cross-link the O-Ag, induces a form of outer membrane stress that compromises the integrity of the S. Typhimurium cell envelope and temporarily renders the bacterium avirulent.
INTRODUCTION
Salmonella enterica serovar Typhimurium (S. Typhimurium) is a Gram-negative enteropathogenic bacterium that causes acute gastroenteritis in humans and typhoid-like fever in mice (19, 21). Attachment to and invasion of intestinal epithelial cells is the pivotal event in S. Typhimurium's infection process. Attachment is facilitated by flagella, which propel S. Typhimurium through viscous environments like the intestinal mucus (37). Uptake of S. Typhimurium into enterocytes, as well as Peyer's patch M cells, is mediated by the so-called Salmonella pathogenicity island 1 (SPI-1) type 3 secretion system (T3SS), a supramolecular “syringe” on the bacterial surface that transfers virulence-associated proteins (effectors) directly into host cells (7, 15, 19, 24, 55). Both flagellum-based motility and the secretion of proteins through the SPI-1 T3SS are energy-dependent processes, requiring the bacterial proton motive force (PMF) and/or cellular ATP stores for full activity (3, 14).
The host's immune response to S. Typhimurium infection is correspondingly complex, involving aspects of the innate and adaptive immune systems in both the mucosal and systemic compartments (17, 21, 61). In the intestinal lumen, the most measurable response to infection is the appearance of secretory IgA (SIgA) antibodies directed against S. Typhimurium surface antigens, notably flagella and the O antigen (O-Ag). While the exact contribution of antiflagellar antibodies to intestinal immunity remains unresolved, it is well established that SIgA antibodies against O-Ag are protective (22, 33, 39, 40). This was first demonstrated experimentally by Michetti and colleagues, who showed that transport of Sal4, a polymeric IgA monoclonal antibody (MAb) against the Salmonella O5 antigen, into the intestinal tracts of mice was sufficient to protect the animals against an otherwise lethal oral dose of virulent S. Typhimurium (39). The same investigators subsequently demonstrated that incubation of S. Typhimurium with Sal4 blocked the ability of the bacterium to invade polarized epithelial cell monolayers in vitro, thereby indicating that Sal4 interferes with the earliest steps in the infection process (40). Because Sal4 is neither bactericidal nor bacteriostatic, it has been proposed that Sal4 must function by “immune exclusion” (40), a phenomenon in which the bacteria are cleared from the intestinal lumen via agglutination and entrapment in mucus. However, the possibility that Sal4 may directly impact certain virulence attributes is now being examined.
Recent work from our laboratory, for example, has revealed that Sal4 interferes with at least two of S. Typhimurium's virulence traits: flagellum-based motility and SPI-1 T3SS-mediated entry into epithelial cells (13). We found that exposure of S. Typhimurium to Sal4 (≥3 μg/ml) in a liquid or agarose environment results in the complete and virtually instantaneous loss of flagellum-based motility. Motility arrest occurred in advance of any detectable signs of microscopic agglutination, indicating that antibody-mediated cross-linking of bacteria did not account for the loss in swimming behavior. At the same time, Sal4-treatment rendered S. Typhimurium noninvasive, even when the need for flagellum-based motility in epithelial cell infection was bypassed by directly promoting bacterium–epithelial-cell contact by low-speed centrifugation. Thus, these data suggest that both flagellar rotation and the secretion through the SPI-1 T3SS are disabled following the binding of Sal4 to the cell surface.
The goal of the present study was to investigate the mechanism by which Sal4 impairs SPI-1 T3SS activity of S. Typhimurium. In light of a recent study that examined the interaction between Shigella flexneri and a protective O-Ag-specific IgA (12), we hypothesize that Sal4 exerts its effects on Salmonella secretion and virulence by inducing changes in bacterial membrane physiology. In accordance with this hypothesis, we provide evidence demonstrating that the exposure of S. Typhimurium to Sal4 results in the inability of the bacterium to secrete proteins into host cells via the SPI-1 T3SS, a concomitant reduction in bacterial membrane energetics, and decreased intracellular levels of ATP. Moreover, Sal4 induced immediate changes in the bacterial outer membrane (OM), as revealed by electron microscopy and lipid permeability studies. Based on these results we propose that Sal4, by virtue of its ability to bind and cross-link the O-Ag, induces a form of OM stress that compromises the integrity of the S. Typhimurium cell envelope and thereby renders the bacterium avirulent.
MATERIALS AND METHODS
Bacterial strains and mammalian cell lines.
All S. Typhimurium strains used in this study are derivatives of strain ATCC 14028 (Table 1). Strains were routinely cultured at 37°C in Luria-Bertani (LB) broth supplemented, when necessary, with kanamycin (50 μg/ml), tetracycline (20 μg/ml), or chloramphenicol (35 μg/ml). James Slauch (University of Illinois at Urbana-Champaign) kindly provided strains JS93, JS481, JS898, and JS899 (9, 27), Hiroshi Nikaido (University of California Berkeley) provided strain HN1140 (44), and Adrianus van der Velden (Stony Brook University) provided strain STN119 (65). Thymine-free M9 medium was supplemented with 0.5% glucose or succinate as carbon sources. HeLa cells were obtained from the Wadsworth Center cell culture core facility and were maintained in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) at 37°C under a 5% CO2-95% air atmosphere.
Table 1.
S. Typhimurium strains used in this study
| Strain | Description | Source or reference |
|---|---|---|
| 14028 | Wild-type S. Typhimurium | ATCC |
| JS93 | zjg8101::Kan oafA126::Tn10d-Tc | 27 |
| JS481 | Δ(invH-avrA)2916::Cm | 9 |
| JS898 | sopB-GSK::Cam | J. Slauch |
| JS899 | slrP-GSK::Kan | J. Slauch |
| STN119 | spiB::mTn5 | 62 |
| HN1139 | phoP102::Tn10d-Cam tolC::Kan | 44 |
| SJF3 | Δrfc::Kan | 13 |
| SJF12 | oafA126::Tn10d-Tc | This study |
| SJF14 | tolC::Kan | This study |
| SJF32 | oafA126::Tn10d-Tc tolC::Kan | This study |
| SJF49 | Δ(invH-avrA)2916::Cam slrP-GSK-Kan | This study |
| SJF52 | Δ(invH-avrA)2916::Cam slrP-GSK | This study |
| SJF53 | Δ(invH-avrA)2916::Cm slrP-GSK spiB::mTn5 | This study |
| SJF61 | oafA126::Tn10d-Tc sopB-GSK-Cam | This study |
| SJF62 | oafA126::Tn10d-Tc slrP-GSK-Kan | This study |
Antibodies and antibody-gold colloid conjugation.
Rabbit polyclonal antisera against Salmonella O antigens (group B factors 1, 4, 5, 12) and H antigens (single factor 2 and factor i) were purchased from BD Difco (Franklin Lakes, NJ). MAb CM 12.1 (IgG2a) against OmpC (57) was a gift from Lynn Bry (Brigham and Women's Hospital, Boston). IgA-secreting hybridomas Sal4 (38) and 23D7 (an isotype control used throughout this study) (34) were maintained as previously described (13). Sal4-colloidal gold immunocomplexes were generated essentially as described by Faulk and Taylor (11) using colloidal gold (5 nm) purchased from Ted Pella (Redding, CA). The conjugation was performed with the assumption that Sal4 has an isoelectric point (pI) of 4.5 to 5.5 (66).
SPI-1 T3S effector translocation assays.
Hemolysis assays using defibrinated whole sheep blood were done as described by Miki et al. (41). For SPI-1 T3SS translocation assays, S. Typhimurium strains expressing chromosomally encoded SopB-glycogen synthase kinase (GSK) and SlrP-GSK fusion proteins were incubated with Sal4 (5 μg/ml) for 15 min before being applied to HeLa cells grown in 6-well microtiter plates. The microtiter plates were subjected to low-speed centrifugation (5 min at a relative centrifugal force of 1,000) to promote bacterium–epithelial-cell contact and then incubated at 37°C for 30 min. Cells were then washed three times in Hanks balanced salt solution (HBSS) to remove unbound bacteria and incubated for an additional hour with gentamicin (100 μg/ml) to kill any remaining extracellular bacteria. HeLa cells were dissociated from the plates by treatment with trypsin, collected by centrifugation, and resuspend in 0.1 ml Tris (10 mM)-EDTA (1 mM) (T10E1). Cells were then mixed with an equal volume of Laemmli sample buffer containing β-mercaptoethanol (5%, vol/vol), boiled for 5 min, and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot analysis with a rabbit monoclonal antibody against GSK-3β (Cell Signaling Technology, Danvers, MA) and horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG secondary antibody (Southern Biotech, Birmingham, AL) in conjunction with ECL Western blotting substrate (Pierce Chemical Co., Rockford, IL). The membranes were then exposed to X-ray film (IBF, Brazil). As the anti-GSK MAb also reacted with endogenous host cell proteins, it was necessary to run a bacteria-only control sample alongside the HeLa cell preparations to distinguish the endogenous GSK from the ectopically expressed GSK.
Measurement of membrane electrical potential.
The electrical potential of S. Typhimurium was measured with the dye 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1) (Invitrogen, Carlsbad, CA), essentially as described by Becker et al. (2). JC-1-loaded bacteria were resuspended in 1 ml of M9 plus 0.5% glucose, pH 7.0. The bacteria were allowed to recover 15 min at room temperature and were then diluted 1:10 into phosphate-buffered saline (PBS) with Sal4 (5 μg/ml), 23D7 (5 μg/ml), or carbonyl cyanide m-chlorophenylhydrazine (CCCP) (100 μM). Treated cells (10 μl) were spotted on microscope slides coated with 1% agarose and then mounted with a coverslip (22 by 50 mm). Bacteria (>100 per treatment) were visualized at room temperature using a Zeiss Axioscope II Plus microscope. Measurements were taken with FITC (excitation, 455 to 495 nm; beam splitter, 500 nm; emission, 505 to 555 nm) and Texas Red filter sets (excitation, 550 to 580 nm; beam splitter, 585 nm; emission, 590 to 650 nm).
Membrane integrity and permeability studies.
To measure the release of specific components of the OM, S. Typhimurium strains were grown to mid-log phase (∼109 CFU/ml), washed twice with PBS, diluted 1:10 into PBS or 100 mM Tris–10 mM EDTA (T100E10), and then treated for 15 min with Sal4 (5 μg/ml) or 23D7(5 μg/ml). The bacteria were then subjected to centrifugation, and the resulting supernatants were filtered through an Acrodisc syringe filter equipped with a 0.2-μm HT Tuffryn membrane (PALL Life Science, Port Washington, NY) to remove any residual cells. The clarified supernatants were spotted onto nitrocellulose membranes (0.45-μm pore size; Bio-Rad) and allowed to air dry at room temperature. The membranes were then incubated overnight at 4°C with a solution of PBS containing 0.5% Tween 20 (PBS-T) and 2% goat or rabbit serum before being probed with rabbit polyclonal antisera (diluted 1:1,000) specific for the O-Ag or H-Ag or with anti-OmpC antibody CM 12.1. The membranes were developed with HRP-labeled secondary antibodies (Southern Biotech) in conjunction with ECL Western blotting substrate (Pierce). The membranes were exposed to X-ray film (IBF, Brazil), which was then digitally photographed using ChemiDoc (Bio-Rad) and subjected to densitometry analysis with Quantity One software (Bio-Rad).
Supernatants were also analyzed for ATP, endotoxin, and alkaline phosphatase. ATP levels were determined using a BacTiter-Glo kit, as recommended by the manufacturer (Promega, Madison, WI). Luminescence was measured using a Spectramax L luminometer (Molecular Devices, Sunnyvale, CA). Lipopolysaccharide (LPS) endotoxin was assayed for with a Limulus amebocyte lysate (LAL) assay (GenScript, Piscataway, NJ). Alkaline phosphatase (AP) was measured utilizing para-nitrophenyl phosphate (PNPP) in a glycine buffer (2 mg/ml) as a substrate. Color change (405 nm) was measured 12 to 20 h later with a Spectramax plate reader (Molecular Devices).
Membrane permeability studies were done essential as described by Murata et al. (44). To prevent active efflux of ethidium bromide (EtBr), we used strains of S. Typhimurium strains lacking TolC. Mid-log-phase cultures of bacteria were diluted 1:10 in sterile water and incubated for 15 min with Sal4 (5 μg/ml) or 23D7 (5 μg/ml), in black 96-well plates with optical bottoms. EtBr (10 μg/ml) was then added to the wells, and the plates were subjected to fluorimetry (excitation, 530 nm; emission, 645 nm) with a Synergy HT fluorometer (Biotek, Winooski, VT) over a period of 10 min.
Transmission electron microscopy (TEM).
For sectioned TEM, mid-log-phase cultures (∼109 CFU/ml) of S. Typhimurium strain 14028 were diluted 1:10 into 50 ml of filtered LB containing 5 μg/ml of Sal4 or Sal4-colloidal gold. Sal4-treated (or control) cells were incubated for 15 min or 60 min at room temperature, after which they were collected by centrifugation and fixed by the addition of glutaraldehyde (2% vol/vol) in cacodylate buffer (pH 7.4). The cells were washed twice in cacodylate buffer to remove excess glutaraldehyde and then resuspended in 1% osmium in cacodylate buffer, subjected to dehydration in an acetone series, and embedded in Epon-Araldite. Semithin (0.20- to 0.25-μm) sections were cut using a Diatome diamond knife on a Reichert Ultracut E ultramicrotome. The sections were stained with uranyl acetate for 20 min and lead for 5 min before being visualized using a Zeiss 910 transmission electron microscope (Carl Zeiss SMT).
For immunogold labeling of whole cells, stationary-phase cultures of S. Typhimurium strain 14028 were collected by centrifugation and then resuspended in 62.5 μl of PBS. A 300-mesh Formvar-coated nickel grid was placed on the drop of bacteria for 1 min and then blotted with Whatman 40 paper to remove excess cells. The cells bound to the grids were subjected to the following: 10 mM NH4 acetate wash, 5 min fixation with 0.75% glutaraldehyde in 100 mM cacodylate acid buffer (pH 7.4), 10 mM NH4 acetate wash, 5 min in 0.02% glycine, 10 min in 1% bovine serum albumin (BSA), 20 min Sal4-gold colloid (3 μg/ml), PBS washes, 10 mM NH4 acetate wash, and counterstaining with 2% ammonium molybdate. Grids were viewed at 80 kV with a Zeiss 910 transmission electron microscope (Carl Zeiss SMT). Images were taken with a Scientific Instruments and Applications, Inc. (SIA), model AAC charge-coupled device (CCD) camera. Drops (∼40 μl) of all solutions were placed on dental wax.
For cryoelectron microscopy, mid-log-phase cultures (∼109 CFU/ml) of S. Typhimurium strain 14028 were collected by centrifugation, resuspended in an equal volume of filtered LB, and then diluted 1:5 into PBS, pH 7.4. Sal4 (5 μg/ml) or an equivalent volume of PBS was added to the cells, which were incubated for 15 min. A drop of cells was spotted onto carbon-coated copper grids, plunge-frozen in liquid ethane, and imaged at −176°C using a JEM-4000FX electron microscope equipped with a Gatan GIF 2002 energy filter.
Statistical analysis.
Statistical analysis (Student's t test, one-way analysis of variance [ANOVA]) was carried out with Excel 2008 for Mac (Microsoft, Redmond, WA) and GraphPad Prism version 5.00 for Mac OS X (GraphPad Software, San Diego, CA).
RESULTS
SPI-1-mediated T3SS activity is suppressed upon exposure of S. Typhimurium to Sal4.
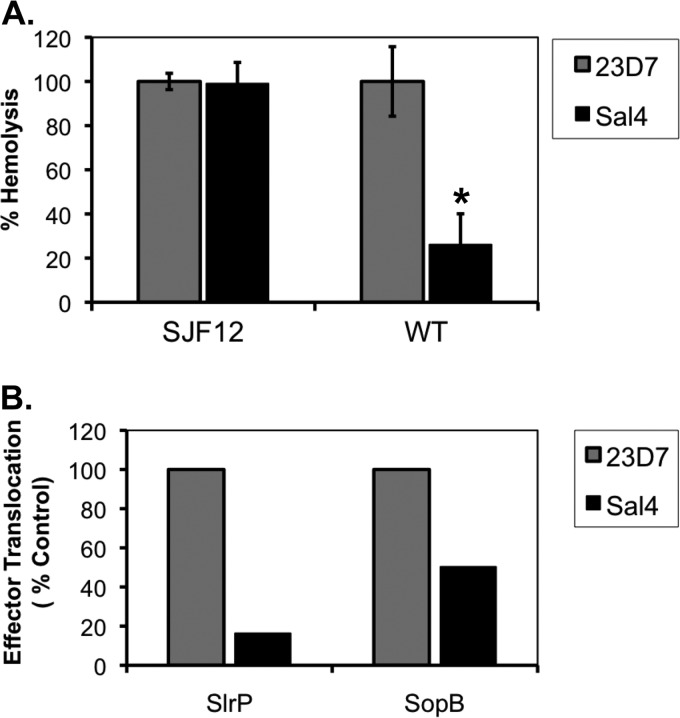
To examine more directly the possibility that Sal4 inhibits SPI-1 T3SS activity in S. Typhimurium, we compared the ability of control and Sal4-treated bacteria to induce hemolysis of sheep red blood cells (RBCs), a well-established measure of SPI-1-mediated pore formation (41). We observed that the hemolytic activity of Sal4-treated wild-type S. Typhimurium was reduced by >75%, compared to that of bacteria treated with the isotype control antibody, 23D7 (Fig. 1A). In contrast, the hemolytic activity of an isogenic oafA strain of S. Typhimurium, known as SJF12, was unaffected by the addition of Sal4 (Fig. 1A). SJF12 lacks the Sal4 epitope due the loss of the integral membrane transacylase encoded by oafA, which is required to terminally modify the O-Ag (58, 59).
Fig 1.
Reduced activity of the SPI-1 T3SS in the presence of Sal4. (A) Hemolysis as an indirect reporter of SPI-1 T3SS activity. Strains SJF12 and 14028 (WT) were incubated with Sal4 (5 μg/ml) or the ricin toxin-specific isotype control MAb 23D7 (5 μg/ml) for 15 min before the addition to 50% sheep whole blood, as described in Materials and Methods. SJF12 lacks the O5 epitope due to a chromosomal mutation in the oafA gene, which encodes a membrane-integral acetylase (58). The amount of hemolysis associated with each experimental condition was expressed as a percentage of the activity of the wild type or oafA strains alone. The data are the averages (with standard errors [SE]) from a single representative experiment done in triplicate. Sal4 treatment resulted in a statistically significant (*, P < 0.05) reduction in hemolytic activity, as determined by the Student t test. (B) Delivery of SPI-1 T3SS effector proteins into host cells. S. Typhimurium strains JS899 and JS898 expressing the chromosomally encoded effector protein SlrP or SopB, respectively, fused to GSK, were tested in HeLa cell invasion assays in the presence of Sal4 (5 μg/ml) or 23D7 (5 μg/ml), as described in Materials and Methods. Western blots of HeLa cell lysates probed with a GSK-specific antibody were subjected to densitometry. The values plotted on the y axis (“effector translocation”) are relative to the amount of GSK detected in HeLa cells infected with strain 14028 in the absence of either 23D7 or Sal4.
To examine SPI-1 T3SS-dependent translocation directly, S. Typhimurium strains expressing chromosomally encoded effector proteins SlrP and SopB fused to GSK were tested in HeLa cell invasion assays, in the absence or presence of Sal4. SlrP is a E3 ubiquitin ligase for mammalian thioredoxin and is secreted by SPI-1 (as well as SPI-2) (4, 10, 56). SopB, a SPI-1 effector, is a phosphoinositide phosphatase involved in the formation of salmonella-containing vesicles (1). We found that translocation of SlrP-GSK and SopB-GSK into the host cell was reduced by ∼80% and ∼55%, respectively, upon treatment of S. Typhimurium with Sal4, compared to that of bacterial cells treated with the isotype control antibody, 23D7 (Fig. 1B). The amount of SlrP translocation mediated by Sal4-treated S. Typhimurium (∼20%) was roughly equivalent to that of strain SJF53 (∼18%), which lacks both the SPI-1 and SPI-2 T3SS (data not shown). Translocation of SlrP-GSK and SopB-GSK by the oafA mutant of S. Typhimurium was unchanged upon addition of Sal4 (data not shown), demonstrating that recognition of the O-Ag by Sal4 must occur for the antibody to interfere with SPI-1 secretion.
In addition to being injected into host cells during infection, the SPI-1 effector proteins are secreted into culture supernatants at low levels by S. Typhimurium, irrespective of the presence of host cells (30). To examine whether Sal4 has an impact on this basal level of effector secretion, we monitored by Western blot analysis the extracellular and intracellular levels of SlrP-GSK from S. Typhimurium grown in the presence or absence of Sal4. Sal4 had no effect on extracellular or intracellular SlrP levels under any growth condition examined (e.g., 4 h with aeration or 16 h without aeration) (data not shown). We conclude from these experiments that Sal4 suppresses the injection of effector proteins into host cells but not the general synthesis of the effector proteins themselves.
Impact of Sal4 on membrane potential and ATP production.
The observation that exposure of S. Typhimurium to Sal4 results in a coincident loss of flagellum-based motility and SPI-1 T3SS led us to examine the possibility that Sal4 treatment influences the PMF. In Salmonella and other Gram-negative bacteria, the PMF is the membrane-localized electrochemical gradient that powers flagellar rotation, T3SS, and ATP synthesis (3, 52, 68). The PMF consists of differences in electrical membrane potential (ΔΨ) and proton concentration (ΔpH). In S. Typhimurium, the best measure of the ΔpH is motility, as flagellar motor speed varies linearly with the PMF gradient. We previously reported that Sal4 arrests S. Typhimurium flagellum-based motility within a matter of minutes, a finding that is strongly indicative of Sal4's having an impact on ΔpH (13).
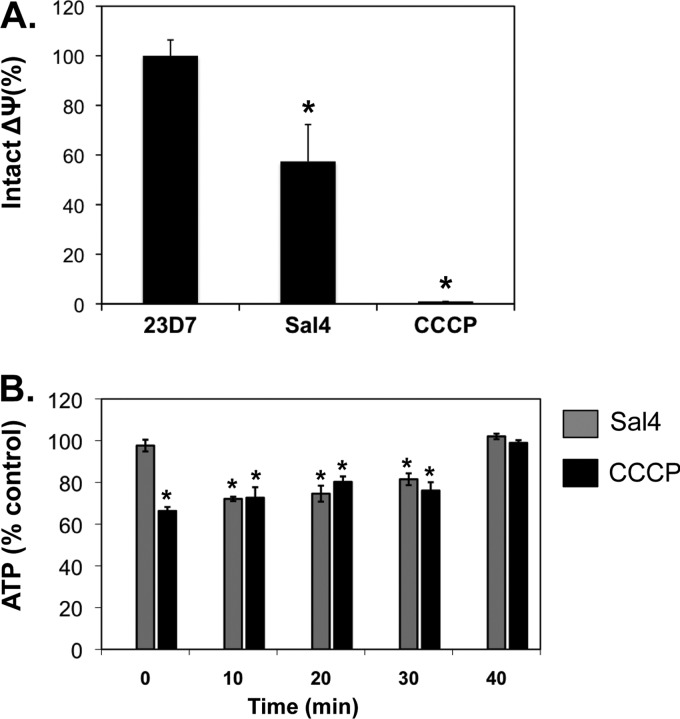
To determine the effect of Sal4 on the ΔΨ component of PMF, mid-log-phase cultures of S. Typhimurium were loaded with JC-1, a cationic dye whose fluorescent emission serves as an indirect measure of the electrical membrane potential (2, 25), and then exposed to 23D7 (5 μg/ml), Sal4 (5 μg/ml), or CCCP (100 μM). The monoclonal IgA antibody 23D7 served a negative control for these studies, while the proton ionophore CCCP served as a positive control. CCCP at a concentration of 100 μM has been shown to effectively deplete the PMF of S. Typhimurium (2). Indeed, we confirmed that CCCP (100 μM) treatment of S. Typhimurium resulted in a complete loss of ΔΨ (Fig. 2A). Sal4 (5 μg/ml) treatment, by comparison, resulted in a partial loss of the PMF, as evidenced by an ∼40% decrease in the number of red cells 15 min following antibody exposure (Fig. 2A). In contrast, there was no change in the PMF following treatment with the control antibody 23D7.
Fig 2.
Reduced membrane potential and ATP levels in response to Sal4. (A) Mid-log-phase cells of S. Typhimurium were loaded with the dye JC-1 and then treated with Sal4 (5 μg/ml) or CCCP (100 μM) for 15 min. The samples were then visualized immediately by fluorescence microscopy, as described in Materials and Methods. The number of red (intact potential) and green (depleted potential) fluorescent bacteria in each sample was determined. The values obtained from the PBS-treated control cells were set to 100%. Each column represents the average value (with standard error) obtained from a minimum of three experiments. Significance, as determined using the Student t test, is indicated by asterisks: *, P < 0.05. (B) ATP levels in S. Typhimurium decline following treatment with Sal4. Mid-log-phase cultures of S. Typhimurium were diluted 1:10 into microtiter plates containing M9 minimal medium with Sal4 (5 μg/ml) or CCCP (100 μM) and incubated for 15 min before being assayed for ATP. Data are the percentage of ATP relative to wild-type S. Typhimurium treated with the isotype control antibody 23D7. Statistically significant differences (P < 0.05) between experimental and control samples, as determined by the Student t test, are indicated by asterisks.
As a corollary to these studies, we also examined Sal4's impact on ATP levels in S. Typhimurium. As expected, CCCP (100 μM) induced an immediate reduction (>30%) in total ATP levels, which returned to normal levels by 40 min (Fig. 2B). Sal4 treatment also resulted in reduced ATP levels (>25%), which rebounded within 30 to 40 min. These data demonstrate that exposure of S. Typhimurium to Sal4 results in rapid reduction in the PMF, as well as a decrease in intracellular ATP levels, which could account for the coincident loss in SP-1 T3SS activity (Fig. 1) and flagellum-based motility (13).
Sal4 compromises the integrity of the lipid component of S. Typhimurium's OM.
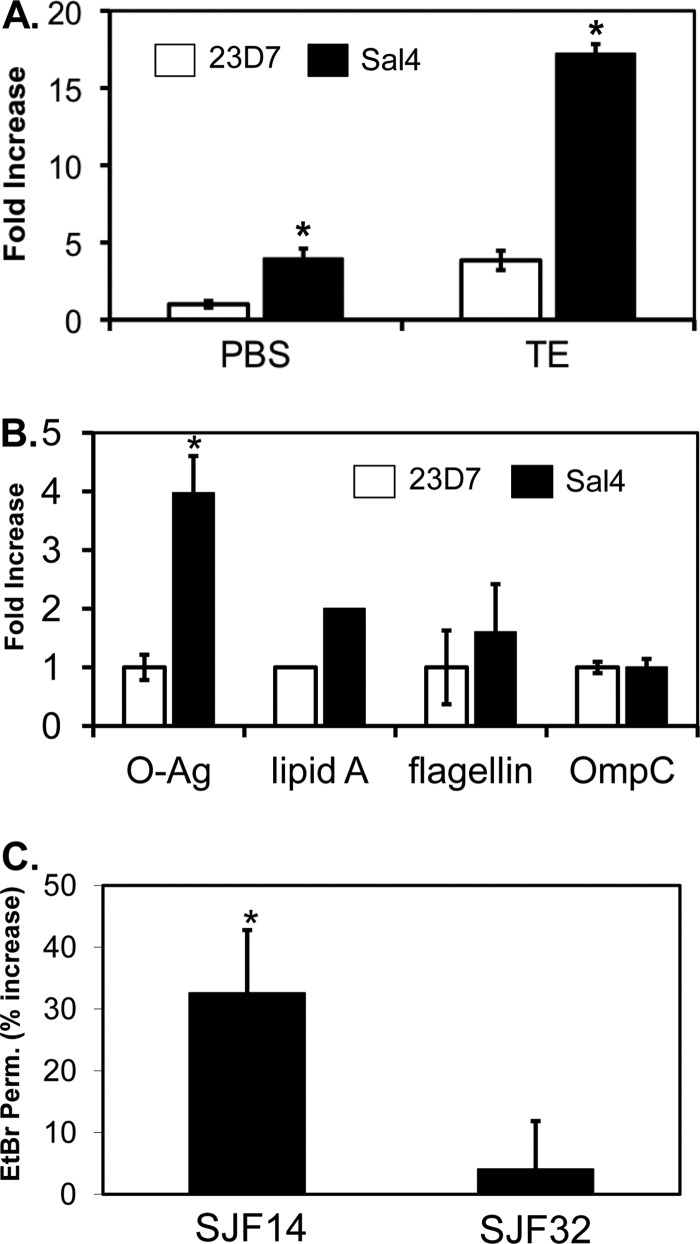
We hypothesized that the coincident loss of flagellum-based motility, SPI T3SS activity, and membrane potential that occurs following exposure of S. Typhimurium to Sal4 could be the consequence of antibody-induced stress to the bacterial OM, as manifested by the shedding of LPS, O-Ag or other OM components into the bacterial supernatants. To test this hypothesis, levels of O-Ag and lipid A in culture supernatants following Sal4 treatment were determined by dot blotting and LAL assay. We observed a 4-fold increase in O-Ag and a 2-fold increase in lipid A levels in supernatants from Sal4-treated cells, compared to parallel cultures treated with the isotype control antibody, 23D7 (Fig. 3A and B). The amount of O-Ag in the external milieu following Sal4 treatment was roughly equivalent to that elicited by T100E10, a solution known to perturb OM integrity (29, 63). When S. Typhimurium was exposed to Sal4 (5 μg/ml) and T100E10 simultaneously, there was an ∼18-fold increase (over baseline) of O-Ag in cell supernatants (Fig. 3A), suggesting that the two stressors work synergistically to destabilize the outer leaflet of the OM. Surprisingly, however, Sal4 did not stimulate a significant release of flagellin or OmpC into culture supernatants (Fig. 3B), indicating that antibody-mediated O-Ag release from the cell surface occurred without shearing of the flagella or release of OM protein components.
Fig 3.
Sal4 enhances the release of the O-Ag from the OM of S. Typhimurium. (A) Mid-log-phase cultures of S. Typhimurium were resuspended in PBS or T100E10 at pH 7.4 and then treated with 23D7 or Sal4 (5 μg/ml) for 15 min. Supernatants were then probed for O-Ag by dot blotting, as described in Materials and Methods. The membrane blots were analyzed by densitometry, and the data (fold increase) are presented relative to PBS control-treated cells. (B) Cell supernatants of S. Typhimurium treated with 23D7 or Sal4, as described for panel A, were subjected to immunoblotting with antibodies against O-Ag, flagellin, or OmpC. Lipid A levels in bacterial supernatants were detected using the LAL assay. Data are averages (with standard errors) from a single experiment done in triplicate. (C) Permeability of the bacterial OM to EtBr following Sal4 treatment. Mid-log-phase cultures of S. Typhimurium SJF14 (tolC::kan) or SJF32 (oafA126::Tn10d-Tc tolC::Kan) were treated with Sal4 (5 μg/ml) for 15 min and then incubated with EtBr (10 μg/ml) for 10 min. Cells were then subjected to quantitative fluorimetry. Baseline permeability values were obtained by measuring EtBr uptake in SJF14 and SJF32 treated with the isotype control antibody 23D7. The percent increase in permeability is defined as the amount of EtBr uptake in Sal4-treated cells divided by the amount obtained from 23D7-treated cells. Significance, as determined using the Student t test, is indicated by asterisks: *, P < 0.05.
To determine whether Sal4-induced loss of O-Ag affects the integrity of the S. Typhimurium OM, we compared the growth and viability of Sal4-treated cells to control cells in response to sublethal concentrations of hydrophobic antibiotics (rifampin, novobiocin, and erythromycin; 10 μg/ml), complement, cationic peptides and defensins (HNP-1, Cryp-4), anionic detergents (SDS), and bile salts. We found that Sal4-treated cells were no more sensitive to the compounds tested than were control cells (data not shown), indicating that the integrity of the O-Ag barrier is not sufficiently affected by Sal4 treatment to allow passage of antimicrobial agents across the OM. On the other hand, Sal4-treated cells were ∼30% more permeable to EtBr than were control cells (Fig. 3C). The ability of EtBr to penetrate the cell envelope is an indirect measure of the integrity of the OM lipid barrier (44). Thus, Sal4 appears to perturb the lipid A aspect of LPS but not to alter the barrier function of the O-Ag.
To further elucidate the impact of Sal4 on the lipid portion of the OM, we assayed bacterial supernatants for the presence of the periplasmic protein alkaline phosphatase (AP) from a strain of S. Typhimurium ectopically expressing the Escherichia coli phoA gene (18). We found that AP levels were ∼3-fold higher in the supernatants of Sal4-treated cells than in those of control cells (data not shown), suggesting that antibody binding to the O-Ag induces localized alterations in lipid membrane permeability. Sal4's effects on the cell envelope were localized to the OM, because there was no detectable increase of β-galactosidase or ATP in culture supernatants following antibody treatment (data not shown). β-Galactosidase (when expressed from a gene fusion in S. Typhimurium) and ATP are cytoplasmic markers that are extruded from cells only when the integrity of the bacterial inner membrane (IM) is compromised. Collectively, these data suggest that Sal4 selectively alters the integrity of the OM lipid bilayer without grossly affecting the barrier functions of the O-Ag or IM.
Ultrastructural changes in the OM of S. Typhimurium following Sal4 exposure.
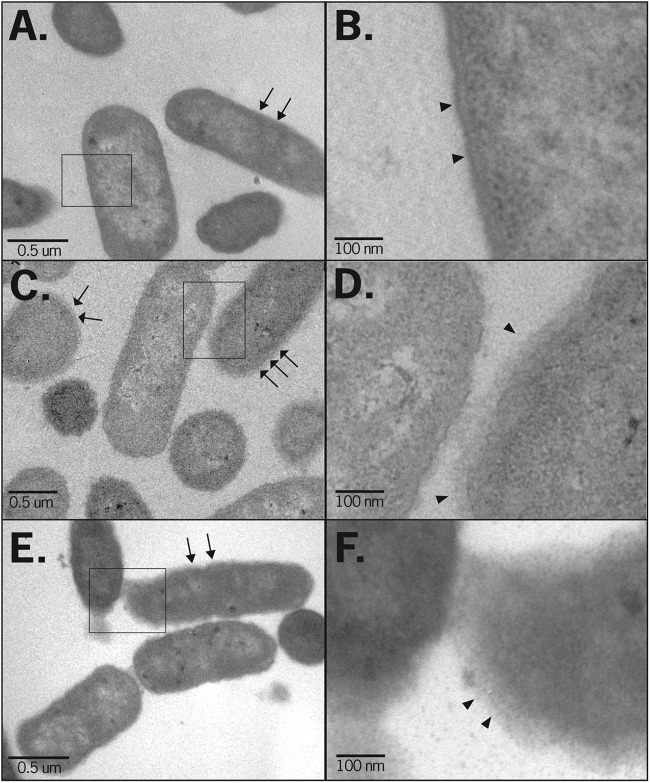
To examine Sal4's impact on the bacterial OM at the ultrastructural level, control and Sal4-treated S. Typhimurium cells were subjected to TEM. The outer surfaces of control cells were relatively smooth and well defined (Fig. 4A and B). In contrast, the surfaces of Sal4-treated cells were surrounded by an ∼50-nm-thick “fuzzy coat” and occasionally had an undulating appearance that was suggestive of membrane damage (Fig. 4C and D). Interestingly, Sal4's impact on the ultrastructure of the bacterial envelope became more pronounced with time, as evidenced by the fact that 1 h after antibody treatment there was an almost complete a loss in definition of S. Typhimurium's outer and inner membranes (Fig. 4E and F). Moreover, the bacteria became mucoid and appeared to be surrounded by a structure that is reminiscent of a capsular exopolysaccharide (EPS).
Fig 4.
Sal4 causes distortion of the bacterial envelope. S. Typhimurium strain 14028 was treated with PBS (A and B) or Sal4 (5 μg/ml) for 15 min (C and D) or 60 min (E and F) and then collected by centrifugation, fixed with glutaraldehyde, and processed for TEM, as described in Materials and Methods. The OM of control cells (arrows [A] or arrowheads [B]) were uniformly smooth. In contrast, cells treated with Sal4 for 15 min were surrounded by a fuzzy coat (arrows [C] or arrowheads [D]). The fuzzy coat was more pronounced after 60 min incubation with Sal4 (arrows [E] or arrowheads [F]). The boxed areas in panels A, C and E are enlarged in panels B, D, and F, respectively.
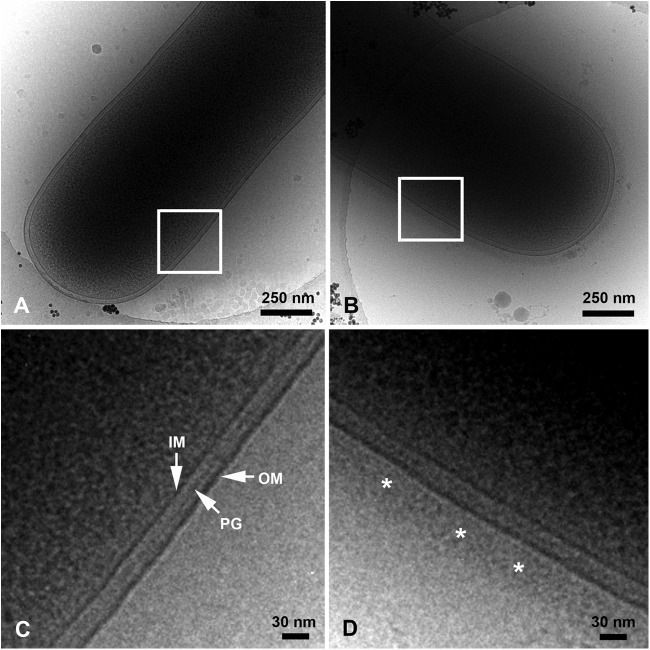
To further examine the impact of Sal4 on the surface topology of S. Typhimurium, bacteria were subjected to cryo-electron microscopy (cryo-EM). Cryo-EM enables the imaging of bacterial cells at near-native state, free of both the fixation and sectioning artifacts that are typically associated with conventional TEM (49). Analysis of control cells by cryo-EM enabled resolution of the IM and OM, as well as the peptidoglycan layer (Fig. 5A and C). Control cells were uniformly surrounded by a distinct layer of low-electron-density material (∼15 to 20 nm thick) that likely corresponds to the O-Ag. In contrast, Sal4-treated cells were surrounded by an ∼50- to 60-nm-thick halo that was similar in appearance to the fuzzy coat observed by conventional TEM (Fig. 5B and D). No such halo was evident around cells of the S. Typhimurium oafA mutant, strain SJF12, following treatment with Sal4 (data not shown), indicating that the observed alterations in the OM of wild-type cells was dependent on Sal4 binding to the O-Ag.
Fig 5.
Cryo-electron micrographs of S. Typhimurium following treatment with Sal4. Control (PBS-treated [A and C]) or Sal4-treated (5 μg/ml for 15 min [B and D]) wild-type S. Typhimurium cells were spotted onto carbon-coated copper grids, plunge-frozen in liquid ethane, and imaged at −176°C using a JEM-4000FX electron microscope. Sal4-treated cells were surrounded by a fuzzy coat ∼50 to 60 nm thick (D, asterisks), which was absent from control cells. IM, inner membrane; PG, peptidoglycan layer; OM, outer membrane. The boxed areas in panels A and B are enlarged in panels C and D, respectively.
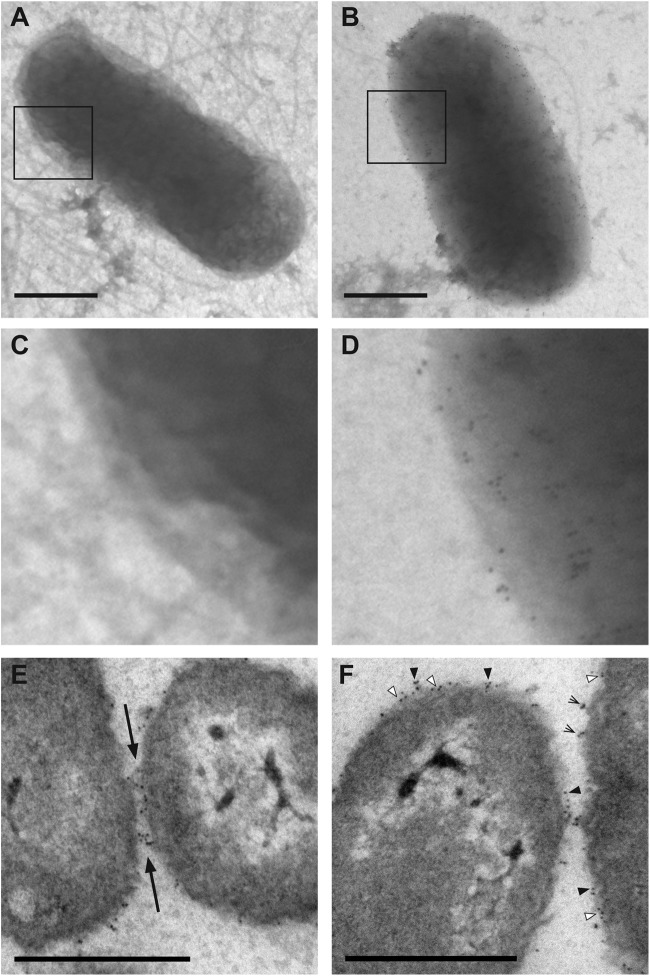
We reasoned that the halo surrounding Sal4-treated cells could be antibody decorating the cell surfaces. To examine this possibility by immunogold labeling, mid-log-phase cultures of 14028 or SJF12 were treated with Sal4-colloidal gold particles (5 nm; 3 μg/ml) for 15 min, washed, spotted on Formvar-coated copper grids, and visualized by TEM. As expected, there were no Sal4-colloidal gold particles detected on the surfaces of SJF12 cells, whereas a multitude of particles were present on the surfaces of wild type cells (Fig. 6A to D). Quantitative analysis indicated that there were an average of ∼440 Sal4-gold particles per bacterium, which corresponds to <0.1% of the total O-Ag epitopes being bound by antibody. Analysis of thin sections revealed that Sal4-colloidal gold particles were often associated with the EPS-like material surrounding the bacteria, as well as with the EPS-like material bridging two adjacent cells (Fig. 6E and F). Individual colloidal gold particles were occasionally observed adjacent to the OM, suggesting that Sal4 is capable of “penetrating” the O-Ag (Fig. 6F), although we cannot exclude the possibility that some of these particles may have penetrated the OM as a result of fixation or processing. Nonetheless, these images clearly demonstrate that Sal4 is associated with the fuzzy coat but that the antibody itself cannot account for the halo associated with antibody-treated cells.
Fig 6.
Association of Sal4 with the O-Ag of S. Typhimurium. (A to D) Mid-log-phase cultures of S. Typhimurium strain SJF12 (A and C) or 14028 (B and D) were spotted on Formvar-coated grids, fixed with glutaraldehyde, and then treated with Sal4-gold colloid (3 μg/ml) for 15 min. The grids were then visualized using a Zeiss 910 electron microscope, as described in Materials and Methods. As expected, Sal4-gold particles did not bind to the surface of SJF12 cells, which lack the O5 epitope due to a mutation in the oafA gene. In contrast, Sal4-gold particles were distributed uniformly around strain 14028 cells. The boxed areas in panels A and B are enlarged in panels C and D, respectively. (E and F) Mid-log-phase cultures of S. Typhimurium strain 14028 were treated with Sal4-gold colloid (5 μg/ml) for 15 min, collected by centrifugation, fixed with glutaraldehyde, and processed for TEM, as described in Materials and Methods. (E) Gold particles were often concentrated at sites of cell adhesion (Earrows). (F) Sal4-gold particles tended to be clustered on the surface (black arrowheads) but were occasionally embedded in the OM (white arrowheads) and situated at sites of membrane detachment (lined arrows). These observations suggest that Sal4 may both penetrate and promote the detachment of the LPS layer. Bars, 0.5 μm.
DISCUSSION
The O-Ag covers more than 75% of the surface of S. Typhimurium (48). It is therefore not surprising that the O-Ag is a primary target of SIgA following Salmonella infections (39, 59). The mechanisms by which these antibodies interfere with invasion of intestinal epithelial cells by S. Typhimurium, however, are not well understood. It is increasingly apparent that “immune exclusion” cannot fully account for SIgA's contribution to mucosal immunity, which has led us to propose that antibodies like Sal4 must have additional effector functions beyond simply promoting bacterial clearance through agglutination and entrapment in mucus (12, 13, 23, 33, 35, 40). In accordance with this hypothesis, we recently reported that Sal4 (under nonagglutinating conditions) is a rapid and potent inhibitor of S. Typhimurium flagellum-based motility (13). In the present study, we now put forth evidence that Sal4 has immediate effects on T3SS activity, membrane energetics, and OM integrity. Based on these results, we propose that Sal4, by virtue of its ability to bind and cross-link the O-Ag, induces a form of OM stress that in turn triggers signal transduction and/or physiochemical pathways that render (at least temporarily) S. Typhimurium avirulent.
We found that exposure of S. Typhimurium to Sal4 for as little as a 15 min resulted in a significant reduction in SPI-1 T3SS activity, as demonstrated by an ∼80% decrease in SPI-1-dependent pore formation, as well as the diminished delivery of SPI-1 effector proteins into host cells. We previously argued that Sal4 is unlikely to sterically hinder S. Typhimurium from attaching to host cells or host cell receptors, due to the fact that Sal4 is nominally 28 nm in length and would not be expected to project sufficiently far from the bacterial surface to interfere with the T3SS needle, which is estimated to be as much as 80 nm in length (5, 42, 69). On the other hand, we cannot exclude the possibility that Sal4 indirectly impacts the capacity of the T3SS to be activated, particularly in light of the fact that antibody binding appears to promote the formation of an EPS-like substance (fuzzy coat). It has been reported that an increase in O-Ag length can effectively shield the SPI-1 T3SS (20).
It is intriguing to speculate that the loss of SPI-1 T3SS activity following Sal4 treatment may in fact be due to a reduction in membrane potential. It is now well established that the PMF and ATP are required to fully energize T3SS in Gram-negative pathogens (14, 52, 69). We observed that Sal4-treated cells were affected in both ΔpH, as evidenced by a loss in flagellum-based motility (13), and ΔΨ, as revealed by a fluorescent imaging of JC-1-loaded cells. Moreover, cytoplasmic ATP levels declined within minutes of antibody treatment. Unlike the proton ionophore CCCP, however, Sal4 has no demonstrable bacteriostatic or bactericidal activity (13, 39). Thus, Sal4's impact on membrane potential would have to be sufficient to disable flagellar rotation and T3SS activity, without adversely impacting growth or viability. This is not implausible, considering that others have established that flagellar motor speed, for example, varies linearly with the PMF (3).
Although the O-Ag is firmly anchored via lipid A to outer leaflet of the OM, Gram-negative bacteria like E. coli and S. Typhimurium are known to shed LPS and other membrane components in response to physical and chemical stresses (29, 31, 32, 63, 64). In this study, we found that Sal4 treatment of S. Typhimurium resulted in an ∼3- to 4-fold increase in the amount of LPS present in bacterial supernatants, corresponding to ∼2% of release of the total LPS from cell surfaces. Sensitizing the OM of S. Typhimurium by concomitant treatment with TE augmented Sal4's effect by a factor of almost 5. Sal4-mediated damage to the cell envelope appeared to be confined to the lipid component of the OM, as release of LPS into culture supernatants was not accompanied by a corresponding increase in flagella or OMPs. In addition, there was no direct evidence of OM blebbing, thereby demonstrating that Sal4-induced membrane damage does not involve stress-induced shedding of OM vesicles, as others have described (36). In fact, the observation that Sal4-treated cells were more permeable to EtBr than control cells, but not more sensitive to antibiotics, complement, cationic peptides (defensins), anionic detergents, or bile salts, further argues that Sal4 specifically affects the lipid A component of the OM and not the O-Ag per se. This is seemingly counterintuitive, considering that Sal4's target is the O-Ag itself. Nonetheless, there is evidence that stabilization of the LPS leaflet and lipid barrier is achieved in part through alignment of neighboring O-Ag side chains (43, 47). Thus, antibody-induced cross-linking of the O-Ag could theoretically impact the integrity of the lipid barrier.
TEM and cryo-EM analysis revealed that Sal4-treated bacteria are surrounded by an electron-dense material that we refer to as a fuzzy coat. This electron-dense material was evident as early as 15 min after antibody exposure and became more pronounced with time. Although the exact nature of this material remains unknown, we speculate that it likely constitutes one or more polysaccharides. At early time points, for example, the fuzzy coat could represent alternative conformations of the O-Ag. The O-Ag is thought to be highly dynamic in nature, assuming coiled and various extended conformations (26, 28, 67). In an extended (“hair standing on end”) conformation, the O-Ag would be expected to form a halo ∼50 to 60 nm thick, which is roughly equivalent to the thickness of the fuzzy coats observed surrounding antibody-treated cells. Furthermore, Sal4, by virtue of the fact that it is polyvalent, is theoretically capable of simultaneously engaging four neighboring O-Ag subunits, possibly “locking” the O-Ag in an extended conformation. Based on our immunogold labeling studies, we estimate that there were ∼440 molecules of Sal4 per bacterium in the TEM experiments. Assuming that there are ∼3.5 × 106 LPS molecules per cell, with each LPS molecule having 10 to 30 individual O-Ag subunits (47), the amount of Sal4 used in these studies is sufficient to occupy <0.1% of O-Ag of a single cell. Therefore, it is unclear whether there is sufficient antibody to account for the uniformity of the fuzzy coat.
Alternatively, Sal4 binding to the bacterial OM could trigger the de novo production of long LPSs and/or EPSs. S. Typhimurium is known to synthesize very long LPS (>100 O-Ag repeat units) under certain environmental conditions, such as those encountered during infections (45, 46). In addition, serovars of S. Typhimurium produce at least three types of extracellular polysaccharides, including an O-Ag capsule, cellulose, and colonic acid (8, 16, 50, 51, 60). EPS production by S. Typhimurium is generally associated with the formation of an extracellular matrix and has been implicated in environmental and host persistence, biofilm formation, and immune evasion. Besides temperature, growth phase, and osmolarity, the specific signals involved in triggering EPS production are largely unknown. It is therefore intriguing to speculate that S. Typhimurium may produce one or more EPSs in response to Sal4, possibly as a strategy to evade the mucosal immune response by shedding antibody from the cell surface.
From the data presented in this and previous studies, we propose a model in which Sal4, by virtue of its ability to cross-link the O-Ag, destabilizes the outer leaflet of the OM, thereby perturbing the local integrity of the cell envelope. Localized changes in OM tension could theoretically trigger the gating of mechanosensitive (MS) channels situated in the IM, which in turn would result in a rapid efflux of ions (including protons) and solute from the cell (6, 53). MS channel activation could account for the observed loss in PMF and ATP that we observed in Sal4-treated S. Typhimurium cells. Additionally, we propose that Sal4 may trigger one or more extracytoplasmic stress responses and/or transmembrane signaling pathways that ultimately control the expression of genes involved in virulence and invasion (53, 54). It is even possible that Sal4 represents a unique environmental cue that alerts S. Typhimurium to the fact that it is in an unfavorable host. In preliminary studies, we have observed that prolonged exposure (>24 h) of S. Typhimurium to Sal4 results in an avirulent phenotype and biofilm formation (J. Amarasinghe, R. D'Hondt, and N. Mantis, unpublished data). Thus, while Sal4's effects on membrane integrity would be expected to be relatively short-lived, the response to antibody exposure may have a lasting impact on pathogenesis. The capacity of Sal4 to render S. Typhimurium (at least transiently) avirulent, coupled with its ability to promote bacterial agglutination and clearance via immune exclusion, may explain in large part the antibody's protective capacity in vivo.
ACKNOWLEDGMENTS
We thank Hiroshi Nikaido (University of California, Berkeley), Adrianus van der Velden (Stony Brook University), and James Slauch (University of Illinois Urbana-Champagne) for providing us with bacterial strains. We also acknowledge Elizabeth McCarthy and the Wadsworth Center's Electron Microscopy Core Facility for technical assistance.
This work was supported in part by grants R01HD061916 to N.J.M. from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and 3P41RR001219 to C.A.M. from the National Center for Research Resources (NCRR).
We declare no conflicts of interest.
Footnotes
Published ahead of print 2 April 2012
REFERENCES
- 1. Bakowski MA, et al. 2010. The phosphoinositide phosphatase SopB manipulates membrane surface charge and trafficking of the Salmonella-containing vacuole. Cell Host Microbe 7:453–462 [DOI] [PubMed] [Google Scholar]
- 2. Becker LA, Bang IS, Crouch ML, Fang FC. 2005. Compensatory role of PspA, a member of the phage shock protein operon, in rpoE mutant Salmonella enterica serovar Typhimurium. Mol. Microbiol. 56:1004–1016 [DOI] [PubMed] [Google Scholar]
- 3. Berg HC. 2003. The rotary motor of bacterial flagella. Annu. Rev. Biochem. 72:19–54 [DOI] [PubMed] [Google Scholar]
- 4. Bernal-Bayard J, Ramos-Morales F. 2009. Salmonella type III secretion effector SlrP is an E3 ubiquitin ligase for mammalian thioredoxin. J. Biol. Chem. 284:27587–27595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blocker A, Komoriya K, Aizawa S. 2003. Type III secretion systems and bacterial flagella: insights into their function from structural similarities. Proc. Natl. Acad. Sci. U. S. A. 100:3027–3030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Booth IR, Edwards MD, Black S, Schumann U, Miller S. 2007. Mechanosensitive channels in bacteria: signs of closure? Nat. Rev. Microbiol. 5:431–440 [DOI] [PubMed] [Google Scholar]
- 7. Cossart P, Sansonetti PJ. 2004. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304:242–248 [DOI] [PubMed] [Google Scholar]
- 8. de Rezende CE, Anriany Y, Carr LE, Joseph SW, Weiner RM. 2005. Capsular polysaccharide surrounds smooth and rugose types of Salmonella enterica serovar Typhimurium DT104. Appl. Environ. Microbiol. 71:7345–7351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellermeier CD, Ellermeier JR, Slauch JM. 2005. HilD, HilC and RtsA constitute a feed forward loop that controls expression of the SPI1 type three secretion system regulator hilA in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 57:691–705 [DOI] [PubMed] [Google Scholar]
- 10. Ellermeier CD, Slauch JM. 2003. RtsA and RtsB coordinately regulate expression of the invasion and flagellar genes in Salmonella enterica serovar Typhimurium. J. Bacteriol. 185:5096–5108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faulk WP, Taylor GM. 1971. An immunocolloid method for the electron microscope. Immunochemistry. 8:1081–1083 [DOI] [PubMed] [Google Scholar]
- 12. Forbes SJ, Bumpus T, McCarthy EA, Corthesy B, Mantis NJ. 2011. Transient suppression of Shigella flexneri type 3 secretion by a protective O-antigen-specific monoclonal IgA. mBio 2(3):e00042–11 doi:10.1128/mBio.00042-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Forbes SJ, Eschmann M, Mantis NJ. 2008. Inhibition of Salmonella enterica serovar Typhimurium motility and entry into epithelial cells by a protective antilipopolysaccharide monoclonal immunoglobulin A antibody. Infect. Immun. 76:4137–4144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Galan JE. 2008. Energizing type III secretion machines: what is the fuel? Nat. Struct. Mol. Biol. 15:127–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Galan JE, Wolf-Watz H. 2006. Protein delivery into eukaryotic cells by type III secretion machines. Nature 444:567–573 [DOI] [PubMed] [Google Scholar]
- 16. Gibson DL, et al. 2006. Salmonella produces an O-antigen capsule regulated by AgfD and important for environmental persistence. J. Bacteriol. 188:7722–7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Griffin AJ, McSorley SJ. 2011. Development of protective immunity to Salmonella, a mucosal pathogen with a systemic agenda. Mucosal Immunol. 4:371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gunn JS, Belden WJ, Miller SI. 1998. Identification of PhoP-PhoQ activated genes within a duplicated region of the Salmonella typhimurium chromosome. Microb. Pathog. 25:77–90 [DOI] [PubMed] [Google Scholar]
- 19. Haraga A, Ohlson MB, Miller SI. 2008. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 6:53–66 [DOI] [PubMed] [Google Scholar]
- 20. Holzer SU, Schlumberger MC, Jackel D, Hensel M. 2009. Effect of the O-antigen length of lipopolysaccharide on the functions of Type III secretion systems in Salmonella enterica. Infect. Immun. 77:5458–5470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hsu HS. 1989. Pathogenesis and immunity in murine salmonellosis. Microbiol. Rev. 53:390–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Iankov ID, et al. 2004. Protective efficacy of IgA monoclonal antibodies to O and H antigens in a mouse model of intranasal challenge with Salmonella enterica serotype Enteritidis. Microbes Infect. 6:901–910 [DOI] [PubMed] [Google Scholar]
- 23. Iankov ID, Petrov DP, Mladenov IV, Haralambieva IH, Mitov IG. 2002. Lipopolysaccharide-specific but not anti-flagellar immunoglobulin A monoclonal antibodies prevent Salmonella enterica serotype Enteritidis invasion and replication within HEp-2 cell monolayers. Infect. Immun. 70:1615–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jones B, Ghori N, Falkow S. 1994. Salmonella typhimurium initiated murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J. Exp. Med. 180:15–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jovanovic G, Lloyd LJ, Stumpf MP, Mayhew AJ, Buck M. 2006. Induction and function of the phage shock protein extracytoplasmic stress response in Escherichia coli. J. Biol. Chem. 281:21147–21161 [DOI] [PubMed] [Google Scholar]
- 26. Kastowsky M, Gutberlet T, Bradaczek H. 1992. Molecular modelling of the three-dimensional structure and conformational flexibility of bacterial lipopolysaccharide. J. Bacteriol. 174:4798–4806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim ML, Slauch JM. 1999. Effect of acetylation (O-factor 5) on the polyclonal antibody response to Salmonella typhimurium O-antigen. FEMS Immunol. Med. Microbiol. 26:83–92 [DOI] [PubMed] [Google Scholar]
- 28. Labischinski H, et al. 1985. High state of order of isolated bacterial lipopolysaccharide and its possible contribution to the permeation barrier property of the outer membrane. J. Bacteriol. 162:9–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Leive L. 1965. Release of lipopolysaccharide by EDTA treatment of E. coli. Biochem. Biophys. Res. Commun. 21:290–296 [DOI] [PubMed] [Google Scholar]
- 30. Lin D, Rao CV, Slauch JM. 2008. The Salmonella SPI1 type three secretion system responds to periplasmic disulfide bond status via the flagellar apparatus and the RcsCDB system. J. Bacteriol. 190:87–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Loeb MR. 1974. Bacteriophage T4-mediated release of envelope components from Escherichia coli. J. Virol. 13:631–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Loeb MR, Kilner J. 1978. Release of a special fraction of the outer membrane from both growing and phage T4-infected Escherichia coli B. Biochim. Biophys. Acta 514:117–127 [DOI] [PubMed] [Google Scholar]
- 33. Mantis NJ, Forbes SJ. 2010. Secretory IgA: arresting microbial pathogens at epithelial borders. Immunol. Invest. 39:383–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mantis NJ, McGuinness CR, Sonuyi O, Edwards G, Farrant SA. 2006. Immunoglobulin A antibodies against ricin A and B subunits protect epithelial cells from ricin intoxication. Infect. Immun. 74:3455–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mantis NJ, Rol N, Corthesy B. 2011. Secretory IgA's complex roles in immunity and mucosal homeostasis in the gut. Mucosal Immunol. 4:603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McBroom AJ, Kuehn MJ. 2007. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol. Microbiol. 63:545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McGuckin MA, Linden SK, Sutton P, Florin TH. 2011. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 9:265–278 [DOI] [PubMed] [Google Scholar]
- 38. Michetti P, et al. 1991. Production and use of monoclonal IgA antibodies complexed with recombinant secretory component for passive mucosal protection. Adv. Exp. Med. Biol. 310:183–185 [DOI] [PubMed] [Google Scholar]
- 39. Michetti P, Mahan MJ, Slauch JM, Mekalanos JJ, Neutra MR. 1992. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect. Immun. 60:1786–1792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Michetti P, et al. 1994. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology 107:915–923 [DOI] [PubMed] [Google Scholar]
- 41. Miki T, Okada N, Shimada Y, Danbara H. 2004. Characterization of Salmonella pathogenicity island 1 type III secretion-dependent hemolytic activity in Salmonella enterica serovar Typhimurium. Microb. Pathog. 37:65–72 [DOI] [PubMed] [Google Scholar]
- 42. Minamino T, Pugsley AP. 2005. Measure for measure in the control of type III secretion hook and needle length. Mol. Microbiol. 56:303–308 [DOI] [PubMed] [Google Scholar]
- 43. Morrison DC, Leive L. 1975. Fractions of lipopolysaccharide from Escherichia coli O111:B4 prepared by two extraction procedures. J. Biol. Chem. 250:2911–2919 [PubMed] [Google Scholar]
- 44. Murata T, Tseng W, Guina T, Miller SI, Nikaido H. 2007. PhoPQ-mediated regulation produces a more robust permeability barrier in the outer membrane of Salmonella enterica serovar Typhimurium. J. Bacteriol. 189:7213–7222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Murray GL, Attridge SR, Morona R. 2006. Altering the length of the lipopolysaccharide O antigen has an impact on the interaction of Salmonella enterica serovar Typhimurium with macrophages and complement. J. Bacteriol. 188:2735–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Murray GL, Attridge SR, Morona R. 2003. Regulation of Salmonella typhimurium lipopolysaccharide O antigen chain length is required for virulence; identification of FepE as a second Wzz. Mol. Microbiol. 47:1395–1406 [DOI] [PubMed] [Google Scholar]
- 47. Nikaido H. 2003. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67:593–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nikaido H. 1996. Outer membrane, p 29–47 In Neidhardt FC, Curtiss RI, Ingraham J, Lin ECC, Low KB, Magasanik B, Reznikoff WS, Riley M, Schaechter M, Umbarger HE. (ed), Escherichia coli and Salmonella: cellular and molecular biology, vol 1 ASM Press, Washington, DC [Google Scholar]
- 49. Pilhofer M, Ladinsky MS, McDowall AW, Jensen GJ. 2010. Bacterial TEM: new insights from cryo-microscopy. Methods Cell Biol. 96:21–45 [DOI] [PubMed] [Google Scholar]
- 50. Prouty AM, Gunn JS. 2003. Comparative analysis of Salmonella enterica serovar Typhimurium biofilm formation on gallstones and on glass. Infect. Immun. 71:7154–7158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Prouty AM, Schwesinger WH, Gunn JS. 2002. Biofilm formation and interaction with the surfaces of gallstones by Salmonella spp. Infect. Immun. 70:2640–2649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rathinavelan T, et al. 2010. A repulsive electrostatic mechanism for protein export through the type III secretion apparatus. Biophys. J. 98:452–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rowley G, Spector M, Kormanec J, Roberts M. 2006. Pushing the envelope: extracytoplasmic stress responses in bacterial pathogens. Nat. Rev. Microbiol. 4:383–394 [DOI] [PubMed] [Google Scholar]
- 54. Ruiz N, Silhavy TJ. 2005. Sensing external stress: watchdogs of the Escherichia coli cell envelope. Curr. Opin. Microbiol. 8:122–126 [DOI] [PubMed] [Google Scholar]
- 55. Schlumberger MC, et al. 2005. Real-time imaging of type III secretion: Salmonella SipA injection into host cells. Proc. Natl. Acad. Sci. U. S. A. 102:12548–12553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Sherry AE, et al. 2011. Characterisation of proteins extracted from the surface of Salmonella Typhimurium grown under SPI-2-inducing conditions by LC-ESI/MS/MS sequencing. Proteomics 11:361–370 [DOI] [PubMed] [Google Scholar]
- 57. Singh SP, et al. 1999. Recognition specificity of monoclonal antibodies which protect mice against Salmonella typhimurium infection. Res. Microbiol. 150:385–394 [DOI] [PubMed] [Google Scholar]
- 58. Slauch JM, Lee AA, Mahan MJ, Mekalanos JJ. 1996. Molecular characterization of the oafA locus responsible for acetylation of Salmonella typhimurium O-antigen: OafA is a member of a family of integral membrane trans-acylases. J. Bacteriol. 178:5904–5909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Slauch JM, Mahan MJ, Michetti P, Neutra MR, Mekalanos JJ. 1995. Acetylation (O-factor 5) affects the structural and immunological properties of Salmonella typhimurium lipopolysaccharide O antigen. Infect. Immun. 63:437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Snyder DS, Gibson D, Heiss C, Kay W, Azadi P. 2006. Structure of a capsular polysaccharide isolated from Salmonella enteritidis. Carbohydr Res. 341:2388–2397 [DOI] [PubMed] [Google Scholar]
- 61. Strugnell RA, Wijburg OL. 2010. The role of secretory antibodies in infection immunity. Nat. Rev. Microbiol. 8:656–667 [DOI] [PubMed] [Google Scholar]
- 62. Tsolis RM, et al. 1999. Identification of a putative Salmonella enterica serotype Typhimurium host range factor with homology to IpaH and YopM by signature-tagged mutagenesis. Infect. Immun. 67:6385–6393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vaara M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vaara M, Vaara T. 1983. Polycations as outer membrane-disorganizing agents. Antimicrob. Agents Chemother. 24:114–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. van der Velden AW, Lindgren SW, Worley MJ, Heffron F. 2000. Salmonella pathogenicity island 1-independent induction of apoptosis in infected macrophages by Salmonella enterica serotype Typhimurium. Infect. Immun. 68:5702–5709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Waldo FB, Cochran AM. 1989. Mixed IgA-IgG aggregates as a model of immune complexes in IgA nephropathy. J. Immunol. 142:3841–3846 [PubMed] [Google Scholar]
- 67. West NP, et al. 2005. Optimization of virulence functions through glucosylation of Shigella LPS. Science 307:1313–1317 [DOI] [PubMed] [Google Scholar]
- 68. Wilharm G, et al. 2004. Yersinia enterocolitica type III secretion depends on the proton motive force but not on the flagellar motor components MotA and MotB. Infect. Immun. 72:4004–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Woof JM, Kerr MA. 2006. The function of immunoglobulin A in immunity. J. Pathol. 208:270–282 [DOI] [PubMed] [Google Scholar]