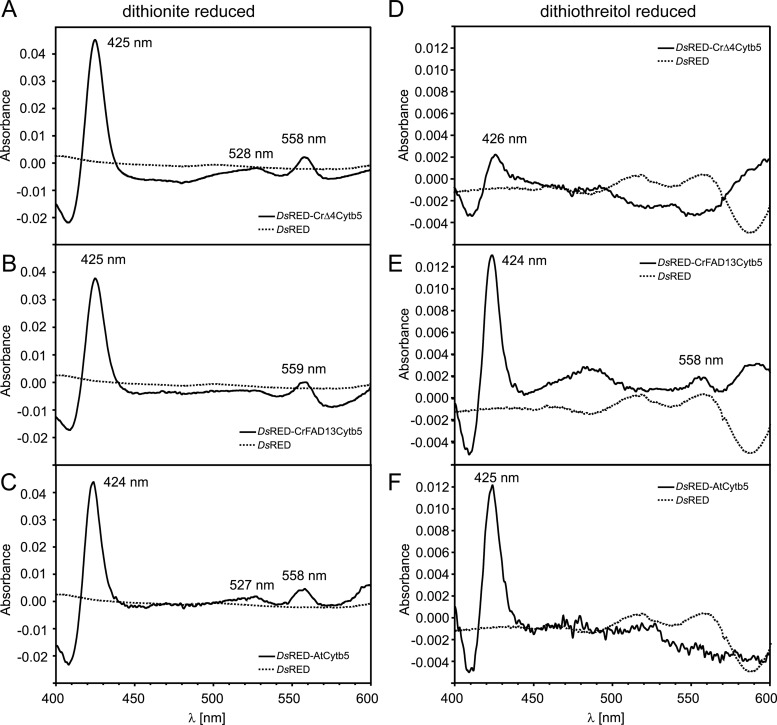
Fig 6.
Redox difference spectra of dithionite-reduced (A to C) and dithiothreitol-reduced (D to F) recombinant cytochrome b5. (A to C) Spectra were calculated from the absorbance of air-oxidized and dithionite-reduced DsRED fusion proteins. DsRED-CrΔ4Cytb5 (A), DsRED-CrFAD13Cytb5 (B), and DsRED-AtCytb5 (C) show difference maxima at 425 (424), 528 (527), and 558 (559) nm, respectively, in line with previous observations (12, 40). (D to F) Spectra were calculated from the absorbance of air-oxidized and DTT-reduced DsRED fusion proteins. The characteristic shape of the cytochrome b5 difference spectrum is still maintained in DsRED-CrFAD13Cytb5 (E) and DsRED-AtCytb5 (F), although the difference between oxidized and DTT-reduced forms is much less pronounced than that with dithionite. In contrast, DsRED-CrΔ4Cytb5 cannot be reduced with DTT (D), indicating that CrΔ4Cytb5 requires a stronger reductant than DTT [ΔE, −330 mV, comparable with that for NADH; ΔE(dithionite), −660 mV].