Fig 3.
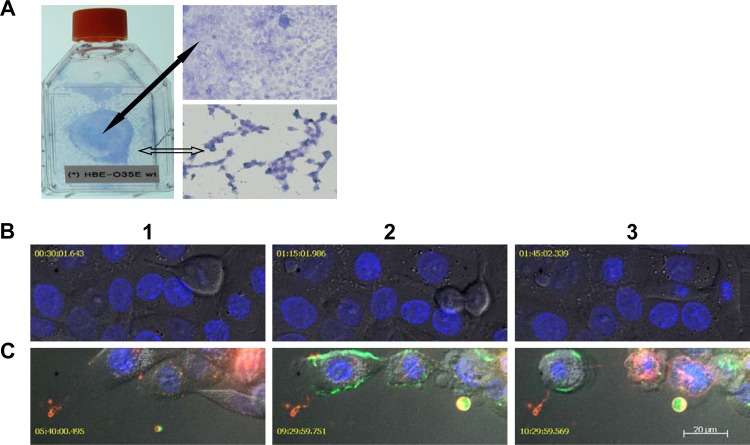
Dividing cells are more susceptible to the cytotoxicity of bacterium-generated NO·. (A) A photo of HBE cells cocultured with M. catarrhalis O35E cells in a T25 tissue culture flask and microscopic (20× objective) images of Giemsa-stained HBE cells in MEM* containing 1 mM nitrite. A fully confluent HBE cell monolayer (indicated by a solid black arrow) was not disrupted, whereas the newly developed HBE cell monolayer (indicated by an open arrow) was severely damaged by M. catarrhalis O35E. (B) Confocal microscopy live-cell images showed a commensal-like relationship of M. catarrhalis and HBE cells cocultured in MEM*. The infection of M. catarrhalis did not have apparent effects on HBE cell division. (C) Confocal microscopy live-cell images show M. catarrhalis exerting pathogenic effects on HBE cells in MEM* containing 1 mM nitrite. Dividing HBE cells failed to divide into two cells and became apoptotic (pseudocolored red) at 3 to 5 h postinfection (panel 1). HBE cells underwent secondary necrosis and released cellular contents, including DNA (YOYO-1 stained DNA green in panels 2 and 3). NO·-induced HBE cell death was associated with apoptotic-cell-like morphological changes (panels 2 and 3). The images in panels B and C were representative images from two replicate experiments. The confocal microscopic images in panels B and C were captured using a 63× objective at different time points after infection. Bar, 20 μm.