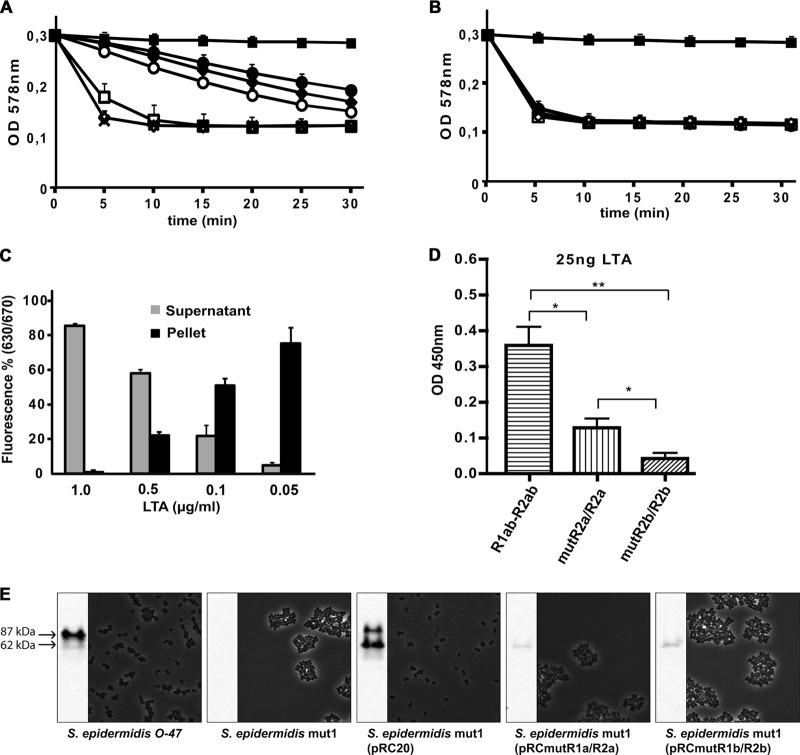
Fig 5.
Interaction of the Atl amidase with PGN and LTA. (A and B) Five micrograms of AM (A) or AM-R1ab-R2ab (B) was incubated with increasing concentrations of LTA (5 to 0.01 μg/ml) prior to addition of PGN. PGN lysis was measured as the decrease of turbidity. AM was not affected by any concentration of LTA tested, while the activity of AM-R1ab-R2ab was decreased at concentrations above 0.1 μg/ml. ×, positive control (PBS); ■, negative control; ●, 1 μg/ml; ♦, 0.5 μg/ml; ○, 0.1 μg/ml; □, 0.05 μg/ml; ♢, 0.01 μg/ml. (C) LTA in different concentrations (0.05 to 1 μg/ml) was preincubated with purified Cy5-R1ab-R2ab prior to addition of PGN. After incubation, PGN was centrifuged, and fluorescence was determined in the pellet and in the supernatant. Increasing concentrations of LTA lead to a decrease in binding of Cy5-R1ab-R2ab to purified PGN. (D) R1ab-R2ab, mutR1a/R2a, and mutR1b/R2b were immobilized on 96-well plates and incubated with purified LTA. Bound LTA was detected by anti-LTA-IgG. Wt repeats are able to bind LTA in high concentrations. Mutation in R1a/R2a leads to reduced binding of LTA, while a mutation in R1b/R2b almost completely prevents binding of LTA. The number of asterisks indicates the degree of significance. (E) Amidase-specific Western blot and light microscopic pictures of S. epidermidis O-47, S. epidermidis mut1, S. epidermidis mut1(pRC20), S. epidermidis mut1(pRCmutR1a/R2a), and S. epidermidis mut1(pRCmutR1b/R2b). Amidase was released from the cell wall by 6% sodium dodecyl sulfate (SDS) and visualized by amidase-specific antibody. The molecular masses correspond to amidase with (87 kDa) and without (62 kDa) propeptide. The error bars indicate standard deviations.