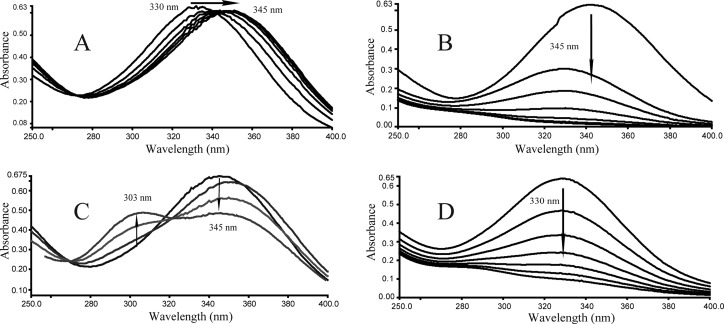
Fig 3.
(A) Isomerization of maleylpyruvate (λmax, 330 nm) to fumarylpyruvate (λmax, 345 nm) catalyzed by H6-BagL. Sample and reference cuvettes contained 10 μM l-cysteine, 50 mM Tris-HCl buffer (pH 8.0), and 4.8 μg of purified H6-BagL in a 1.0-ml mixture. The reaction was initiated by the addition of 50 μM maleylpyruvate. The spectra were recorded every 30 s after the addition of maleylpyruvate. (B) Hydrolysis of fumarylpyruvate by nagK-encoded fumarylpyruvate hydrolase. After the isomerization of maleylpyruvate to fumarylpyruvate, 30 μg of purified H6-NagK was added to both sample and reference cuvettes from the reaction depicted in panel A, and the spectra were recorded every 30 s. (C) Formation of a product with a λmax of ∼303 nm in the presence of excess l-cysteine. The experiment was performed as described for panel A, except the l-cysteine was used at a concentration of 20 μM. (D) Spectrophotometric changes during the transformation of maleylpyruvate catalyzed by both H6-BagL and H6-NagK in the presence of excess l-cysteine. The experiment was performed as described for panel C, except that fumarylpyruvate hydrolase was also present.