Abstract
The stressosome is a 1.8-MDa cytoplasmic complex that conveys environmental signals to the σB stress factor of Bacillus subtilis. A functionally irreducible complex contains multiple copies of three proteins: the RsbRA coantagonist, RsbS antagonist, and RsbT serine-threonine kinase. Homologues of these proteins are coencoded in different genome contexts in diverse bacteria, forming a versatile sensing and transmission module called RST after its common constituents. However, the signaling pathway within the stressosome itself is not well defined. The N-terminal, nonheme globin domains of RsbRA project from the stressosome and are presumed to channel sensory input to the C-terminal STAS domains that form the complex core. A conserved, 13-residue α-helical linker connects these domains. We probed the in vivo role of the linker using alanine scanning mutagenesis, assaying stressosome output in B. subtilis via a σB-dependent reporter fusion. Substitutions at four conserved residues increased output 4- to 30-fold in unstressed cells, whereas substitutions at four nonconserved residues significantly decreased output. The periodicity of these effects supports a model in which RsbRA functions as a dimer in vivo, with the linkers forming parallel paired helices via a conserved interface. The periodicity further suggests that the opposite, nonconserved faces make additional contacts important for efficient stressosome operation. These results establish that the linker influences stressosome output under steady-state conditions. However, the stress response phenotypes of representative linker substitutions provide less support for the notion that the N-terminal globin domain senses acute environmental challenge and transmits this information via the linker helix.
INTRODUCTION
Input (or sensory) domains of bacterial signaling proteins are often joined to output domains by amphipathic α-helices or coiled coils (2, 32, 36). Analysis of such helices should reveal common themes as well as differences regarding interdomain signal transmission. Here we report genetic analysis of a conserved, 13-residue linker that connects the presumed input and output domains in a widely distributed cytoplasmic signaling module, called RST after its three principal protein components (35). The RST module is encoded by diverse bacterial genomes, in contexts that suggest its sensory input can be adapted to control different signaling pathways.
The composition, physical arrangement, and physiological role of the RST module have been most intensively studied in the model Gram-positive bacterium Bacillus subtilis, where it regulates activation of the general stress factor σB in response to a variety of environmental signals, including acid, alcohol, heat, or salt stress (18, 37). B. subtilis RST components were found to form the stressosome (9, 11, 24, 29), a large cytoplasmic complex that consists of multiple copies of the RsbT serine-threonine kinase, the RsbS antagonist protein, and one or more members of the RsbR coantagonist family (where Rsb indicates regulator of sigma-B). In vivo, each stressosome appears to contain a mixture of the paralogous and partly redundant RsbRA, -RB, -RC, and -RD coantagonists (11, 24). Each of these coantagonists has an N-terminal, nonheme globin domain, a 13-residue interdomain linker, and a C-terminal STAS (sulfate transporter/anti-sigma factor antagonist) domain, whereas the smaller RsbS antagonist comprises only a STAS domain (29, 34).
Genetic and biochemical studies have shown that RsbRA, RsbS, and RsbT form a minimal functional stressosome (9, 24). Cryo-electron microscopy images of complexes assembled from purified components suggest an essentially icosahedral structure comprising the STAS domains of 20 RsbRA dimers and 20 RsbS monomers, which directly bind 20 RsbT kinase monomers to the outer surface of the icosahedron (29). The N-terminal, nonheme globin domains of the RsbRA dimers project outward from the core structure and are the presumed route of signal entry into the complex.
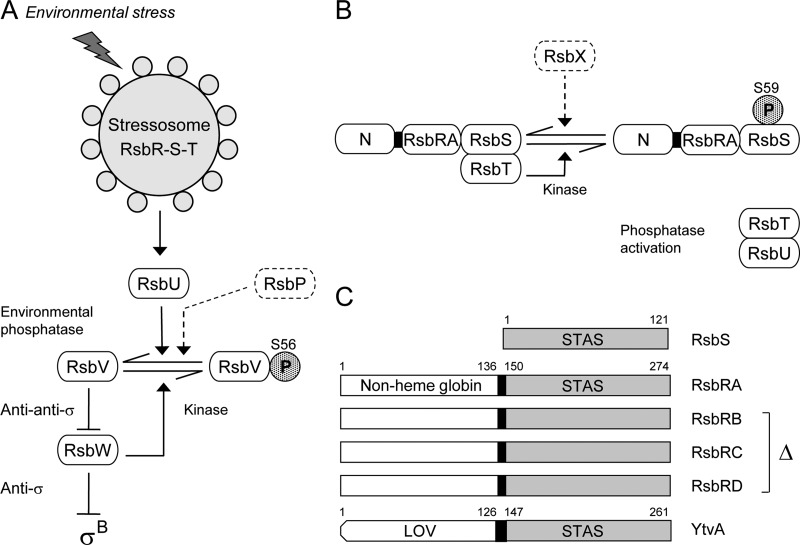
According to the model shown in Fig. 1, in unstressed cells the complex sequesters the RsbT switch protein/kinase and prevents it from binding its regulatory target, the RsbU environmental phosphatase. During the stress response, RsbT phosphorylates the RsbS antagonist on a conserved serine residue; this modification is associated with the release of RsbT from the complex and its subsequent activation of RsbU by direct protein-protein interaction. This basic model is well supported by genetic and biochemical analysis (9, 10, 12, 23, 43). However, the molecular events that promote RsbT release are not well understood. It has been proposed that stress signals elicit a conformational change in the N-terminal input domains of the RsbR coantagonists (29, 34). This change would then be communicated via the central helical linkers to the C-terminal STAS domains in the complex core and, presumably, to the adjacent STAS domain of the RsbS antagonist as well. A shift in core structure is thought to promote both activation of the RsbT kinase and its release from the complex.
Fig 1.
σB Regulatory network. (A) Model of signaling pathways that converge on the RsbV anti-anti-σ and RsbW anti-σ, which directly regulate σB activity. The stressosome controls activation of the RsbU environmental phosphatase in response to diverse signals. The separate RsbP energy phosphatase is not the focus here and is represented only in a dotted outline. Activated RsbU removes the phosphate (stippled P) from RsbV-P, the form found in unstressed cells. RsbV binds RsbW, forcing σB release. Arrowheads indicate activation of protein targets or enzymatic reactions; T-headed lines indicate inhibition. (B) Model of stressosome control of RsbU phosphatase activity. The stressosome comprises the partially redundant RsbRA, -RB, -RC, and -RD coantagonists (represented here as RsbRA, with its N-terminal, nonheme globin domain labeled N) and the RsbS antagonist, which together bind the RsbT kinase. During the stress response, RsbT phosphorylates its RsbS antagonist on S59; RsbT is released to bind and activate RsbU. The RsbX feedback phosphatase (dotted outline) dephosphorylates RsbS-P. (C) The RsbS antagonist has a single STAS domain (residues 1 to 121), whereas the RsbRA coantagonist has a nonheme globin domain (1 to 136) and a STAS domain (150 to 274) joined by the 13-residue helical linker analyzed here (black rectangle). RsbRB, -RC, and -RD coantagonists are structurally similar to RsbRA; these three paralogs were removed from most strains in the study. YtvA is an RsbR family member that positively controls stressosome output in response to blue light, sensed by its LOV domain (residues 1 to 126) and transmitted to STAS (147 to 261) by a 20-residue helical linker (black rectangle).
This route of signal transmission within RsbRA is plausible, but there is no experimental evidence to support it. Although single substitutions within the N-terminal, nonheme globin domain were found to increase system output in unstressed cells, they had no effect on the magnitude of subsequent stress signaling (16). These results indicate that the N-terminal domain influences stressosome function but offer no direct support for the hypothesis that it is a stress sensor. Moreover, little is presently known regarding the function of the 13-residue linker that connects the N-terminal globin to the C-terminal STAS domain (29). Here we use alanine scanning mutagenesis to probe the in vivo operation of this linker, finding that substitutions at different positions had distinct effects on system output in unstressed cells. The periodicity of these effects supports a model in which two linkers associate as parallel paired helices within an RsbRA dimer, with the inside and outside surfaces of the pair making different protein contacts in vivo.
MATERIALS AND METHODS
Bacterial strains and genetic methods.
Standard recombinant methods (40) and natural transformations (13) were used to construct the B. subtilis strains shown in Table S1 in the supplemental material. The plasmids employed in these constructions are listed in Table S2. A QuikChange Lightning kit (Stratagene, La Jolla, CA) was used to introduce missense substitutions into the linker region of the rsbRA gene encoded on integrative plasmid pTG5923 (16); substitution was confirmed by sequencing the entire coding region of each construction. These missense alleles were exchanged for wild-type rsbRA on the chromosome using the I-SceI-mediated method of Janes and Stibitz (20) as described previously (16). The constructed strains also carried a single-copy transcriptional fusion between the ctc promoter and a lacZ reporter to provide an indirect measure of σB activity (8).
β-Galactosidase accumulation assay and Western blots.
Assays were conducted as described previously (16). Briefly, shake cultures were grown at 37°C in buffered Luria broth medium lacking salt (7), with moderate white-light illumination (3 to 4 μmol m−2 s−1). This illumination saturated the blue-light-sensing YtvA positive regulator (5), an RsbR family member and stressosome constituent (17), ensuring that fluctuations in light intensity did not affect assay results. Unstressed samples were taken during early exponential growth up to a cell density of 20 absorbance units (Klett-Summerson colorimeter equipped with a number 66 transmission filter), at which point ethanol or NaCl stress was imposed at a final concentration of 4% (vol/vol) or 0.3 M, respectively. Samples were treated essentially according to the method of Miller (30); β-galactosidase activity was defined as ΔA420 × 1,000 min−1 mg−1 of protein (16). Basal activity was the value measured in unstressed cells at 20 absorbance units; stress activation was the difference between this basal value and the maximum activity realized after stressor addition.
Western blotting, using mouse monoclonal anti-RsbRA antibody provided by William Haldenwang (14), was done as described previously (24). Forty micrograms of protein from wild and mutant cell extracts were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA). Primary and secondary antibody binding was detected using the ECL Plus kit (Amersham Pharmacia Biotech, Piscataway NJ).
Bioinformatic methods.
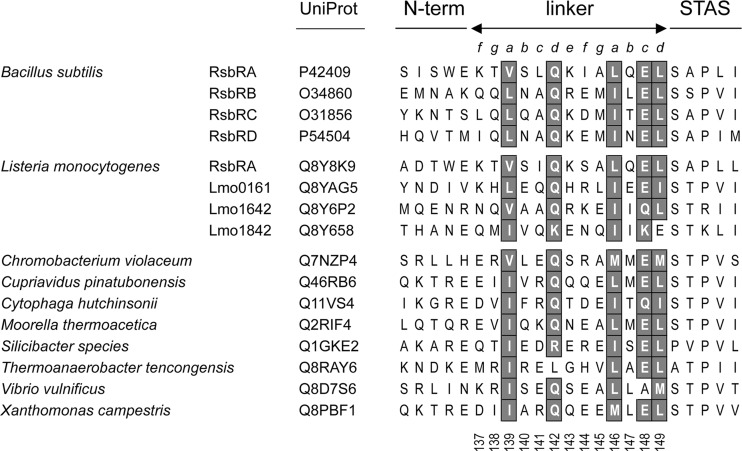
A database of RsbRA homologues was developed using the NCBI BLAST algorithm (1) at default settings. Full-length B. subtilis RsbRA (residues 1 to 274) was queried against the nonredundant database (1 March 2009 version). From the resulting hits, we selected those whose structural genes were encoded in an RST module (38). The list was further reduced by randomly choosing one representative per bacterial or archaeal genus, resulting in the 29 sequences shown in Fig. S1 in the supplemental material. These homologues all had N-terminal domains with a globin fold; some had sequences suggestive of heme binding but most appeared to be nonheme globins, like B. subtilis RsbRA. For the alignment shown in Fig. 2, we created a 16-member data set by combining the eight most diverse linker sequences from Fig. S1 (HHfilter utility from the MPI Bioinformatics Toolkit, http://toolkit.tuebingen.mpg.de/hhfilter) with those of the eight RsbR coantagonist paralogs encoded by the B. subtilis and Listeria monocytogenes genomes (35). Multiple alignment was done using ClustalW with default settings and the BLOSUM 62 matrix (42).
Fig 2.
Conserved residues in diverse RsbR linkers. ClustalW alignment of the linker regions of RsbR paralogs from B. subtilis and L. monocytogenes plus the eight most diverse orthologs from Fig. S1 in the supplemental material, with the conserved positions shaded. Organism, paralog designation, and UniProt identifier indicate linker origin; lines and letters above alignment denote N-terminal and C-terminal domain boundaries and residue position within presumed heptad repeats; vertical numbers below the alignment are B. subtilis RsbRA residue numbers.
Computational modeling.
Superpositions and structural analyses were carried out with EDPDB (44) and visualized and manipulated with the O crystallographic program (21). The 13-residue linker region of B. subtilis RsbRA was modeled as a coiled-coil extension of the globin domain crystal structure (34) (PDB accession number 2BNL). We exploited the similar dispositions of the RsbRA dimer C-terminal and the basic regions of the GCN4/DNA complex model (15) (PDB accession number 1YSA). Initial structural alignments were established by manually docking the 1YSA CD dimer to the 2BNL AB dimer, followed by least-squares superposition of 1YSA residues C254 to C261 and D254 to D261 on 2BNL residues A129 to A136 and B129 to B136 (main-chain root mean square deviation [RMSD] = 1.27 Å). This transformation was used to position the GCN4 coiled-coil region as a helical extension of 2BNL. The equivalent 1YSA residues 262 to 274 were converted to RsbRA linker residues 137 to 149, and the least-hindered side chain rotamers were chosen without further optimization.
Although the continuous helical model is the most parsimonious, other helical registers were investigated by modeling the 13 linker residues in 7 registers (±3 residues from the continuous model, the “0” register). In the 0-register model, three of the five large, nonpolar residues pair at the interface (V139, L146, and L149, along with A145) while two others cluster on the outer face of each helix (L141 and I144). Besides the 0-register, only the −2 register significantly buries some nonpolar residues (L141 and I144) while exposing others (V139, L146, and L149). In all other registers, four or five nonpolar residues were solvent exposed. The 0-register arrangement is in good agreement with the recently determined structure of the Moorella thermoacetica RsbR homolog (39) (PDB accession number 3ZTA), which contains the helical extension (main-chain RMSD of 1.10 Å for RsbRA linker dimer model residues 137 to 149 superimposed on the 3ZTA crystallographic dimer residues 132 to 144).
RESULTS
Linker sequences are conserved at specific positions.
We first compared the sequence of the 13-residue B. subtilis RsbRA linker with orthologs found in diverse RST modules as well as with paralogs encoded separately from these modules, such as the coantagonist paralogs of B. subtilis and L. monocytogenes (35). The N-terminal domains of these proteins all exhibit a globin fold but otherwise share little sequence identity. A multiple alignment of 16 representative linker sequences is shown in Fig. 2. The N-terminal boundary of the linker is set by E136, which marks the last residue of the available nonheme globin structure of B. subtilis RsbRA (34). This glutamate is conserved among the RsbRA orthologs in the figure but less so among the paralogs. The C-terminal boundary is set by the ELSAP motif that forms the junction between the linker and the STAS domain, with SAP (or STP) marking the beginning of STAS (3).
The constancy of the multiple alignment argues for a conserved linker structure, and its heptad periodicity resembles that of α-helical coiled coils. Coiled coils result from the packing of two or more α-helices that manifest a repeat pattern a-b-c-d-e-f-g, in which positions a and d are usually occupied by hydrophobic residues (27). Here the B. subtilis RsbRA linker begins with a lysine (K137) at a presumptive f position. A conserved hydrophobic residue (V139) occupies the first a position, whereas an atypical but highly conserved glutamine (Q142) is found at the first d position. In contrast, conserved hydrophobic residues (L146 and L149) lie at both the second a and d positions. The c position (E148) of this second, incomplete heptad is also highly conserved and marks the beginning of the ELSAP motif that joins the linker and STAS domain. Secondary structure predictions (not shown) suggest that each linker forms an extension of the final α-helix of the N-terminal globin fold, termed the H helix in the B. subtilis RsbRA structure (34). Thus, motion within the N-terminal domain could be transmitted to the STAS domain within the stressosome core by means of this extended helix.
Phenotypes conferred by linker substitutions suggest two separate interacting surfaces.
We performed alanine scanning mutagenesis on 12 of the 13 B. subtilis residues, leaving A145 unexamined. We made additional disruptive substitutions at V139, L146, E148, and L149, which occupy positions conserved in the multiple alignment, and also at K143, whose charge is conserved among all four B. subtilis RsbR paralogs. These disruptive substitutions involved either the replacement of a hydrophobic residue with an arginine (e.g., V139R) or a reversal of charge (e.g., K143E). A two-step procedure was used to exchange the mutant rsbRA alleles encoding each substitution for the wild-type allele on the B. subtilis chromosome. Because the presence of the paralogous RsbRB, -RC, and -RD coantagonists can mask the phenotypes of rsbRA mutations (16), we assayed the effect of each substitution on expression of a σB-dependent reporter fusion in a strain bearing null alleles of rsbRB, rsbRC, and rsbRD.
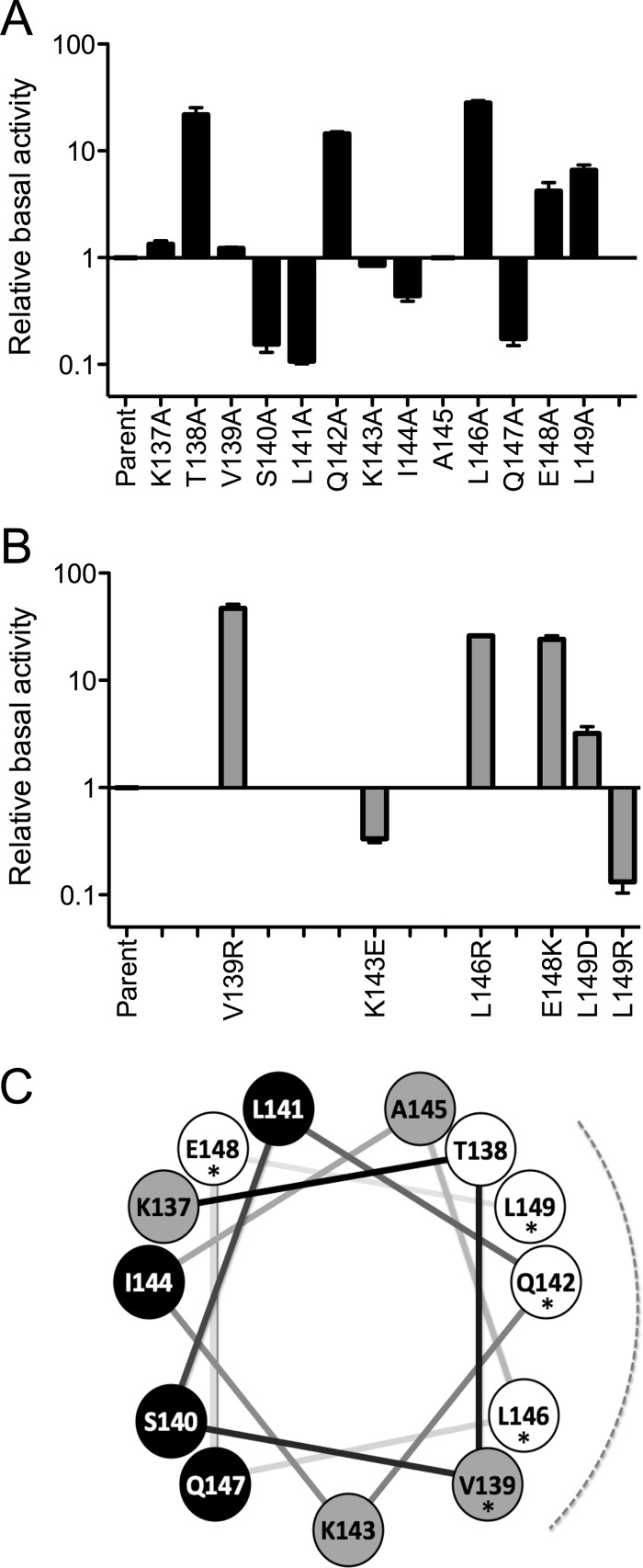
In general, phenotypes elicited by the alanine substitutions were qualitatively similar to those of the more disruptive changes (Fig. 3). Alanine substitution at the conserved Q142, L146, E148, and L149 positions increased σB activity 4- to 30-fold in unstressed cells. However, alanine substitution at the conserved V139 only slightly increased σB activity, while substitution at the more narrowly conserved K143 only slightly decreased it (Fig. 3A). These latter phenotypes became markedly more pronounced in strains bearing the disruptive V139R and K143E substitutions, and they altered σB activity the same direction as the corresponding alanine changes (Fig. 3B). An exception to this qualitative similarity occurred with the L149R substitution, which reversed the elevated phenotype of its L149A counterpart by decreasing σB activity from control levels. To resolve this difference, we made an additional disruptive substitution, L149D, and found it increased σB activity much like L149A. These results indicate that substitutions at L149 can have a range of phenotypes. Nonetheless, alanine substitutions at any of the five conserved residues–V139, Q142, L146, E148, and L149–have the common property of increased system output in unstressed cells.
Fig 3.
Effects of linker substitutions on σB activity in unstressed cells. (A) Relative activity elicited by alanine substitutions, with the parent strain taken as 1 (PB1078, encoding RsbRA as the only coantagonist). β-Galactosidase accumulation from a ctc-lacZ fusion was assayed in logarithmically growing cells; error bars indicate standard errors of the means (SEM) from at least two independent experiments. (B) Relative activity of more disruptive substitutions at conserved positions, with the parent strain taken as 1. (C) Helical wheel representation of the RsbRA linker, with the conserved positions indicated by asterisks, the conserved face by the dotted curve, and the effect of alanine substitution by fill color. Substitutions on the conserved face enhanced output (T138, Q142, L146, L149; white circles), whereas those on the opposite face diminished it (S140, L141, I144, Q147; black circles). Alanine substitutions at the N terminus of the linker (K137) or between the two faces (V139 and K143) had little effect (gray circles). Alanine substitution at E148 was not in accord with this pattern: output was enhanced, but E148 lies on the face opposite the other conserved residues with a similar phenotype.
Notably, alanine substitutions at five of the nonconserved positions also had significant effects. As was the case for the conserved positions, a T138A substitution increased σB activity in unstressed cells by more than 20-fold (Fig. 3A). In contrast, the S140A, L141A, and Q147A substitutions decreased σB activity 6- to 9-fold, whereas I144A decreased it about 2-fold. None of these four restricting alanine substitutions would be expected to disrupt or destabilize the helical structure.
With the exception of E148, the conserved positions at which alanine substitution significantly increased stressosome output lie on one face of a helical wheel representation, and T138 with its similar phenotype is on this same face (Fig. 3C). In contrast, all of the nonconserved positions at which substitution decreased output lie on the opposite face. The two positions at which phenotypes were made plain only with more disruptive substitutions, V139 and K143, lie between these faces. Based on these striking phenotypic patterns, we infer that the linker helix has two discrete surfaces which interact with different partners.
Suppression analysis indicates that mutant RsbRA proteins form functional stressosomes.
The RsbR coantagonists have a negative regulatory role in B. subtilis, and at least one of the four is needed to join with the RsbS antagonist to bind RsbT and keep system output low in unstressed cells (9, 11, 24). By design, our assay strain encoded only the RsbRA paralog. Therefore, substitutions at conserved linker positions might elevate σB activity due to a partial loss of the ability of RsbRA to assemble with RsbS and form functional stressosomes. We used two different approaches to evaluate the properties of mutant RsbRA proteins in vivo.
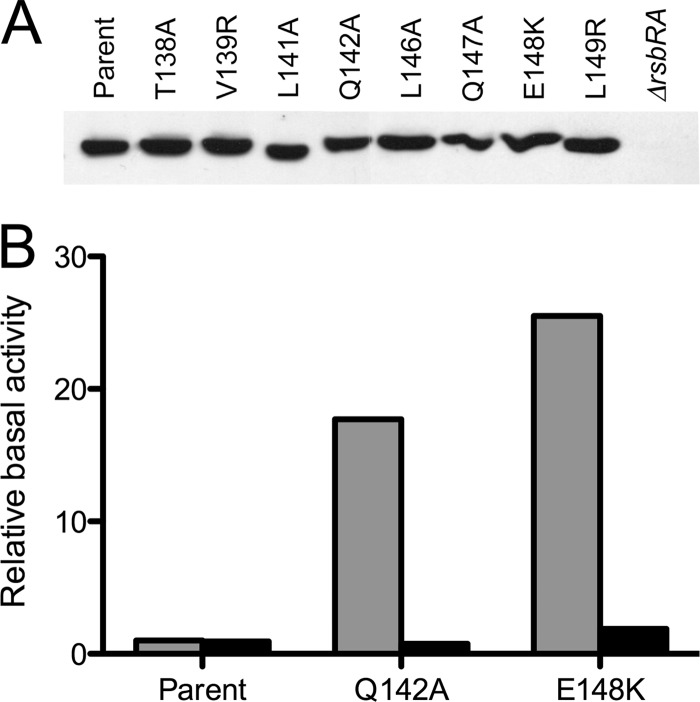
First, Western blotting estimated RsbRA levels in six strains bearing substitutions that strongly increased system output, as well as for two strains with substitutions that significantly decreased it. As shown in Fig. 4A, none of the mutant strains had a steady-state RsbRA level that was noticeably different from the wild-type control. Therefore, elevated σB activity in the six enhancing mutants was not simply due to a decreased amount of RsbRA.
Fig 4.
Representative substitutions did not affect in vivo levels of RsbRA or its ability to form stressosomes. (A) Relative steady-state levels of wild and mutant RsbRA proteins estimated by Western blotting. Extracts of cells bearing designated substitutions were separated by PAGE and probed with anti-RsbRA antibody. (B) Genetic suppression analysis indicates that RsbRA proteins with Q142A and E148K substitutions form functional stressosomes. Relative activities in strains with each designated substitution are indicated by gray bars; activities with the same substitution coupled with the S59A substitution in RsbS are indicated by black bars. Assays were performed on unstressed cells as described in the Fig. 3 legend; activities are expressed with the parent strain taken as 1 (PB1078, encoding RsbRA as the only coantagonist).
Second, genetic suppression tested the functionality of stressosomes formed with mutant RsbRA proteins. The RsbS antagonist requires an RsbR coantagonist to effectively bind RsbT in vitro and prevent high constitutive signaling in vivo (9, 24). Following an environmental stress, the RsbT kinase contributes to the efficiency of its own release from the stressosome by phosphorylating RsbS on serine 59 (9, 25, 28, 43). A phosphorylation-deficient S59A substitution therefore greatly dampens σB activation, but it cannot compensate for the absence of RsbR coantagonist function in sequestering RsbT (23, 24). Beginning with a genetic background in which RsbRA was the only coantagonist present, we combined the allele encoding RsbS-S59A with either Q142A or E148K, representative substitutions that strongly increased system output in unstressed cells. As shown in Fig. 4B, with both RsbRA mutants, the RsbS-S59A allele restored σB activity to the basal level of the parent strain.
This suppression of the mutant phenotype indicates that the tested RsbRA proteins can form stressosomes capable of sequestering RsbT. This result also suggests that the elevated basal level caused by the Q142A or E148K substitution in part reflects abnormally high S59 phosphorylation in unstressed cells. A further indication of the functionality of the mutant stressosomes is their ability to support an environmental stress response, which is described in the next section.
Effect of linker substitutions on stress response.
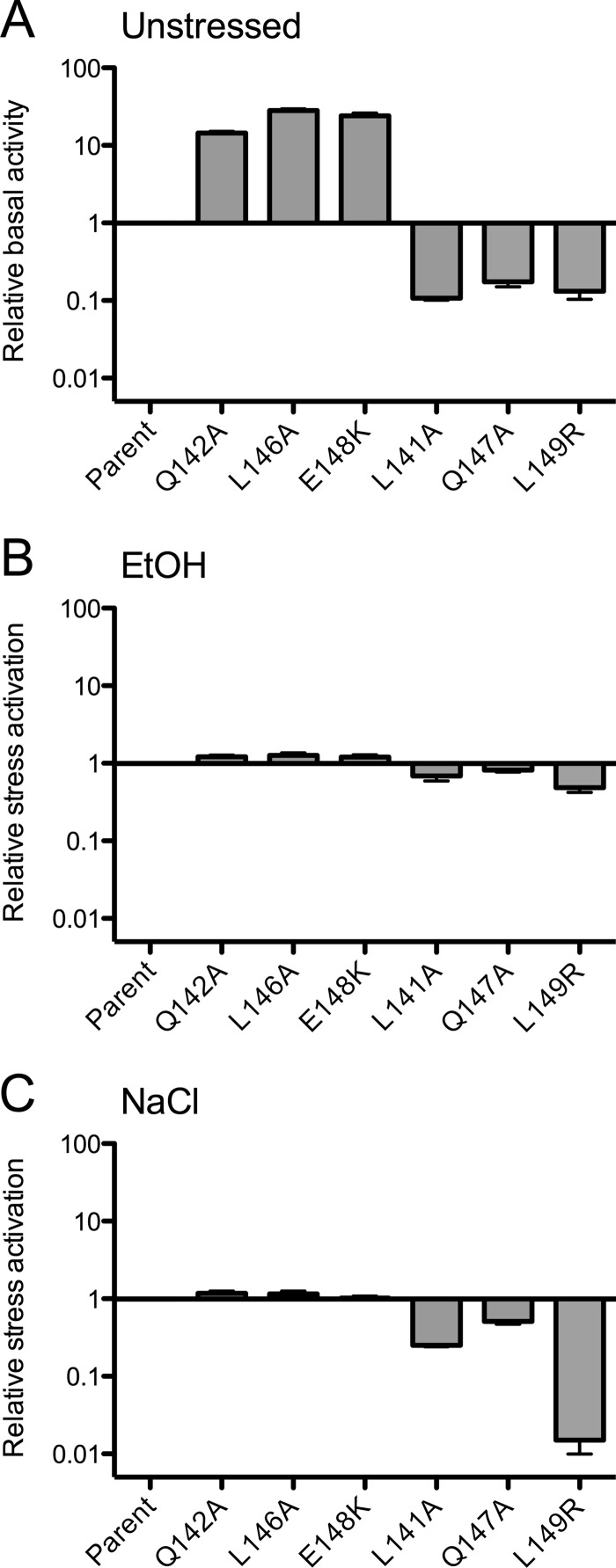
The previous assays addressed the effect of linker substitutions on the steady-state (or basal) output of the complex in unstressed cells. To assess their impact on stress signaling, we determined the phenotypes of representative substitutions in logarithmically growing cells exposed to moderate or mild environmental stresses (4% ethanol or 0.3 M NaCl, respectively).
The Q142A, L146A, and E148K substitutions, which enhanced steady-state output 14- to 30-fold in unstressed cells, had no significant effect on the magnitude of subsequent stress signaling (Fig. 5). These phenotypes resemble those of the previously characterized K82A and K93A substitutions within the N-terminal, nonheme globin domain of RsbRA: elevated basal levels but no effect on stress response (16).
Fig 5.
Effect of representative linker substitutions on stress activation. (A) Data showing relative basal activity in unstressed cells is from Fig. 3, with activity of the parent strain (PB1078, encoding RsbRA as the only coantagonist) taken as 1. Relative activation following 4% ethanol (B) or 0.3 M NaCl (C) stress, with activation of the parent strain taken as 1. Error bars indicate SEM from at least two independent experiments.
The Q147A and L141A substitutions, which restricted output 6- or 9-fold in unstressed cells, also had no significant effect on ethanol stress signaling (Fig. 5). However, these restricting substitutions did reduce the NaCl response by 2- or 4-fold, respectively. Such a diminished response to mild stress probably reflects the lower basal level of σB activity in these strains, which would slow the autocatalytic induction of σB and result in decreased stress sensitivity (19). This consideration cannot fully explain the phenotype of the L149R substitution, which restricted output by a comparable amount in unstressed cells (7-fold) but diminished response to NaCl stress by more than 50-fold (Fig. 5). These restrictive substitutions collectively represent two new classes of rsbRA mutant: significantly reduced basal output with either a modest (Q147A or L141A) or strong (L149R) impact on NaCl response. Notably, only the disruptive L149R substitution had this strong phenotype. Its L149A cousin resembled the Q142A, L146A, and E148K enhancing substitutions, with an elevated basal level (Fig. 3) but no significant effect on stress response (data not shown).
Phenotypes of enhancing substitutions are masked in the presence of other RsbR paralogs.
In the standard assay strain in which the mutant rsbRA allele encoded the only functional coantagonist, the Q142A and E148K substitutions greatly increased basal output compared to the wild-type allele (Fig. 3). However, these strong enhancing phenotypes were completely masked in a strain that also encoded the other three members of the coantagonist family, RsbRB, -RC, and -RD (see Fig. S2 in the supplemental material). Thus, the stressosome complement of the cell must be formed solely from the mutant coantagonist for the phenotype to be revealed.
DISCUSSION
RsbRA and its coantagonist paralogs are thought to comprise the initial sensory and transmission components of a widespread bacterial signaling module, which in B. subtilis forms the σB-activating stressosome (35). Here we used alanine scanning and other missense substitutions to probe the role of a conserved 13-residue linker that connects the presumed input and output domains of RsbRA (29). Our results firmly tie the linker to the control of output levels in unstressed cells but offer less support for a role in conveying signals of acute environmental stress.
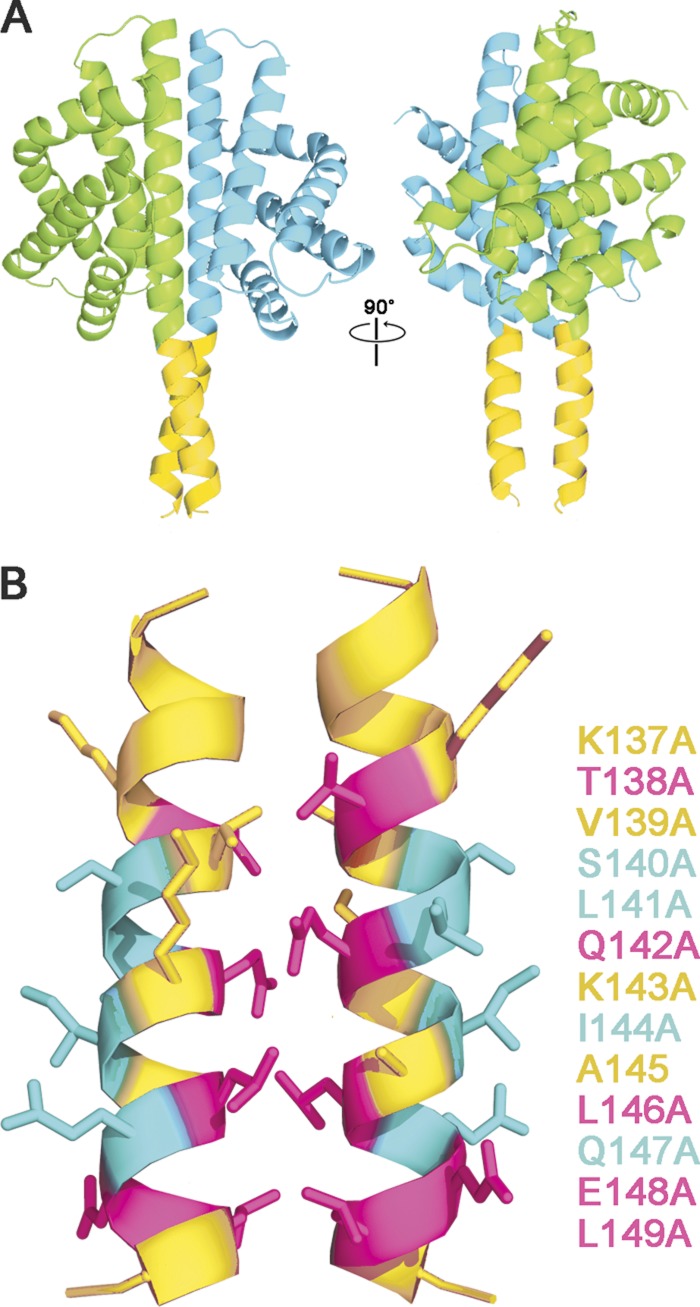
In general, the phenotypes elicited by the alanine substitutions manifested a striking periodicity: those on the conserved face of the helix led to elevated steady-state output from the stressosome, whereas those on the opposite, nonconserved face led to decreased output. We interpret these results to indicate that the two helical faces interact with different partners in vivo. A differential interaction can be most simply explained by the computational model shown in Fig. 6. In this model, the H helices of the globin dimer structure of Murray et al. (34) have been extended to include the adjacent 13-residue linkers, which then form parallel paired helices via their conserved interfacial residues. In this configuration, the nonconserved residues on the opposite face of the linker are free to contact a different binding partner.
Fig 6.
Computational model of paired RsbRA linker helices, derived by extending the H helices of the nonheme globin crystal structure (PDB code 2BNL, chains A and B, cyan and green ribbons) to include the linker helices (gold ribbons, RsbRA residues 137 to 150; see Materials and Methods). (A) Two views of the nonheme globin dimer showing crossover of the linker helices at Q142 (left) and a 90° rotated view looking into the putative interaction surfaces of the paired helices (right). (B) Details of the interaction surfaces, indicating the effects of alanine substitution at positions 137 to 149: substitutions that enhance steady-state output of the stressosome (magenta), those that diminish output (cyan), and those with no significant effect (gold). This correspondence of helical periodicity with mutational phenotype is consistent with the notion that each linker helix interacts with two different binding partners: its counterpart helix via the highly conserved interface residues and an unknown partner via the nonconserved residues on the opposite face.
The model is supported by the similar phenotypes of both N-terminal and linker substitutions that are predicted to affect RsbRA dimerization. The isolated N-terminal, nonheme globin domains of RsbRA form homodimers both in solution and during crystallization (34). Based on the crystal structure, the K82A and K93A substitutions within this domain would be expected to adversely affect homodimer formation, and both substitutions were previously found to share a distinctive phenotype: elevated basal output in unstressed cells but no significant effect on subsequent stress signaling (16). From the computation model shown in Fig. 6, we predict that the Q142A and L146A substitutions tested here would adversely affect interaction of the paired linker helices, and these substitutions had the same characteristic phenotypes as the K82A and K93A globin substitutions. Our genetic results support the notion that the N-terminal region of RsbRA normally functions as a dimer in vivo and further suggest that the dimerization interface encompasses both the nonheme globin domain and the paired linker helices that are extensions of this domain.
The phenotypes caused by alanine substitution at the nonconserved S140, L141, I144, and Q147 residues were unexpected: decreased basal output in unstressed cells, coupled with a modest decrease in stress signaling for the representatives tested. Because none of these substitutions would alter linker helicity, we conclude that the absent side chains provide inter- or intramolecular contacts that contribute to normal stressosome operation. Due to steric and topological considerations, the outer faces of the paired linker helices are unlikely to directly contact the N-terminal, nonheme globin domain. Possible interacting partners therefore include an unknown small molecule or protein, the RsbT kinase, or the nearby STAS domain of RsbRA itself. In the latter case, the nonconserved face of the helix may promote the efficient coupling of the N- and C-terminal domains.
The phenotypes of substitutions at the conserved E148 and L149 residues are at variance with the simplest view of two interacting faces. However, these exceptional phenotypes could signal a different role of these particular residues in coupling the linker and STAS domain. According to the computational model, E148 points away from the helical interface, but E148A and E148K elicited the same distinctive phenotype as substitutions at the Q142 and L146 interface residues: elevated steady-state output with no effect on stress response. This distinguishes E148 from the restricting S140, L141, L144, and Q147 substitutions on the same helical face, suggesting that the E148 side chain contacts a different partner or a different part of the STAS domain. On the other hand, the characteristic elevated phenotype of L149A was consistent with an interface role, but the disruptive L149R phenotype had the opposite phenotype: diminished steady-state output and a severe effect on the response to NaCl stress. We speculate that the arginine side chain effectively jams the linker-STAS interface in a less-responsive conformation.
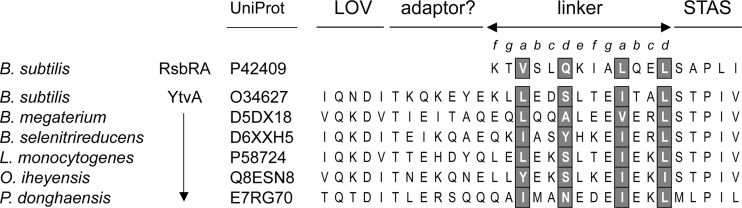
Because full-length RsbRA aggregates in vitro, as yet, there is no complete structure to corroborate these genetic inferences. However, studies of two related proteins are informative. The crystal structure of the N-terminal, nonheme globin domain of the RsbRA ortholog from the Moorella thermoacetica stressosome (PDB accession number 3ZTA) also includes the linker residues (39). This domain shares only 12% sequence identity with its B. subtilis counterpart but is structurally equivalent. Notably, its associated linkers form parallel helices at the dimerization interface, which is in good agreement with the computational model shown in Fig. 6 (see Materials and Methods). Other studies have focused on the RsbRA paralog YtvA. Like RsbRA, YtvA is associated with the B. subtilis stressosome but has no apparent coantagonist function, acting only as a positive signaling element in response to blue light (4, 6, 17). It has a domain organization similar to RsbRA and forms a head-to-head homodimer in solution (22). As shown in Fig. 1, instead of an N-terminal, nonheme globin, YtvA has a light-sensing LOV (light-oxygen-voltage) domain (26, 33) connected to a C-terminal STAS domain via a 20-residue Jα helix, the properties of which suggest formation of a parallel coiled coil in the YtvA homodimer (31). Structural studies indicate that light incidence brings about a small relative rotation of LOV monomers within a dimer crystal (33), and analysis of hybrid proteins suggests that Jα transfers this rotation to an output domain (31). Nuclear magnetic resonance studies of the full-length YtvA homodimer find that the C-terminal region of Jα is less flexible than the central part of the linker, supporting a role in coupling Jα to STAS (22).
The Jα helices of YtvA orthologs have little sequence similarity with the shorter RsbR linkers. However, as shown in Fig. 7, the C-terminal 13 residues of representative Jα helices share a heptad periodicity resembling that of the RsbR linkers, although in Jα a small residue (rather than a glutamine) usually occupies the d position of the first heptad. We speculate (i) that the seven N-terminal residues of Jα serve to adapt it to the LOV domain, (ii) that such an adaptor is unnecessary for the nonheme globin domain of RsbRA, and (iii) that the 13 C-terminal residues serve a similar role in Jα and the RsbR linker: transmitting information from the N-terminal domain to STAS.
Fig 7.
Comparison of the RsbRA linker with Jα linkers from diverse YtvA orthologs. YtvA is an RsbRA-like stressosome component that senses blue light via its N-terminal LOV domain, communicating this to its C-terminal STAS domain by means of a 20-residue Jα helix (see Discussion). The C-terminal 13 residues of representative Jα linkers are aligned with the B. subtilis RsbRA linker, with the conserved positions shaded. Lines and letters above the alignment show N- and C-terminal domain boundaries and the residue position within the presumed heptad repeats.
The YtvA paralog provides a logical prototype of signaling from an N-terminal sensor domain to a C-terminal STAS output domain. However, the question of whether the nonheme globin domain of RsbRA is a sensor of acute environmental stress is unresolved. Characterization of two similar nonheme globin domains that modulate sporulation in Bacillus anthracis suggests that they are capable of sensing fatty acids and chloride via binding within a distinctive tunnel and chamber (41). The lack of these structural features seems to preclude the B. subtilis domain from sensing similar signals, and the cellular parameter to which it responds remains unknown. Furthermore, our genetic analyses of both the RsbRA N-terminal domain (16) and linker (this study) yielded results that are not in keeping with the usual N- to C-terminal route of information transfer within bacterial signaling proteins. Surprisingly, substitutions predicted to disrupt dimerization of the N-terminal region increased stressosome output as much as 30-fold in unstressed cells but had no effect on the magnitude of ethanol or salt response.
At minimum, our results indicate that stressosome signaling has unusual features that remain to be explained. They also raise the possibility of an alternative hypothesis: the N-terminal sensory domains of RsbR family members primarily serve to adjust steady-state output of the stressosome. In the case of YtvA, this adjustment would be in response to blue light, whereas for RsbRA, it would be in response to a cytoplasmic parameter yet to be identified. In this view, the true sensor of acute ethanol or salt challenge lies downstream from the N-terminal, nonheme globin domain. This downstream sensor might comprise the arrangement of C-terminal STAS domains within the icosahedral core of the stressosome, in which stress-influenced shifts of conformational equilibria could directly affect RsbT release by increasing its dissociation rate. The two alternative models, N- or C-terminal domain as the environmental stress sensor, might be distinguished by substitutions in the RsbRA N-terminal region that specifically influence stress response while leaving steady-state output unchanged. The L149R substitution described here does affect stress signaling to a greater extent than steady state. However, due to its equivocal effects and position at the linker-STAS interface, the L149R phenotype is compatible with either model.
Supplementary Material
ACKNOWLEDGMENTS
We thank Lily Yang for her assistance in constructing the linker substitutions, William Haldenwang for providing the anti-RsbRA antibody, and Valley Stewart for helpful comments on the manuscript.
This research was supported by Public Health Service grant RO1 GM42077 from the National Institute of General Medical Sciences.
Footnotes
Published ahead of print 18 May 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anantharaman V, Balaji S, Aravind L. 2006. The signaling helix: a common functional theme in diverse signaling proteins. Biol. Direct 1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aravind L, Koonin EV. 2000. The STAS domain—a link between anion transporters and antisigma-factor antagonists. Curr. Biol. 10:R53–R55 [DOI] [PubMed] [Google Scholar]
- 4. Avila-Pérez M, Hellingwerf KJ, Kort R. 2006. Blue light activates the σB-dependent stress response of Bacillus subtilis via YtvA. J. Bacteriol. 188:6411–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Avila-Pérez M, van der Steen JB, Kort R, Hellingwerf KJ. 2010. Red light activates the σB-mediated general stress response of Bacillus subtilis via the energy branch of the upstream signaling cascade. J. Bacteriol. 192:755–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Avila-Pérez M, et al. 2009. In vivo mutational analysis of YtvA from Bacillus subtilis: mechanism of light activation of the general stress response. J. Biol. Chem. 284:24958–24964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boylan SA, Redfield AR, Brody MS, Price CW. 1993. Stress-induced activation of the σB transcription factor of Bacillus subtilis. J. Bacteriol. 175:7931–7937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boylan SA, Rutherford A, Thomas SM, Price CW. 1992. Activation of Bacillus subtilis transcription factor σB by a regulatory pathway responsive to stationary-phase signals. J. Bacteriol. 174:3695–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen CC, Lewis RJ, Harris R, Yudkin MD, Delumeau O. 2003. A supramolecular complex in the environmental stress signalling pathway of Bacillus subtilis. Mol. Microbiol. 49:1657–1669 [DOI] [PubMed] [Google Scholar]
- 10. Chen CC, Yudkin MD, Delumeau O. 2004. Phosphorylation and RsbX-dependent dephosphorylation of RsbR in the RsbR-RsbS complex of Bacillus subtilis. J. Bacteriol. 186:6830–6836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Delumeau O, Chen CC, Murray JW, Yudkin MD, Lewis RJ. 2006. High-molecular-weight complexes of RsbR and paralogues in the environmental signaling pathway of Bacillus subtilis. J. Bacteriol. 188:7885–7892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delumeau O, et al. 2004. Functional and structural characterization of RsbU, a stress signaling protein phosphatase 2C. J. Biol. Chem. 279:40927–40937 [DOI] [PubMed] [Google Scholar]
- 13. Dubnau D, Davidoff-Abelson R. 1971. Fate of transforming DNA following uptake by competent Bacillus subtilis. I. Formation and properties of the donor-recipient complex. J. Mol. Biol. 56:209–221 [DOI] [PubMed] [Google Scholar]
- 14. Dufour A, Voelker U, Voelker A, Haldenwang WG. 1996. Relative levels and fractionation properties of Bacillus subtilis σB and its regulators during balanced growth and stress. J. Bacteriol. 178:3701–3709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellenberger TE, Brandl CJ, Struhl K, Harrison SC. 1992. The GCN4 basic region leucine zipper binds DNA as a dimer of uninterrupted α helices: crystal structure of the protein-DNA complex. Cell 71:1223–1237 [DOI] [PubMed] [Google Scholar]
- 16. Gaidenko TA, Bie X, Baldwin EP, Price CW. 2011. Substitutions in the presumed sensing domain of the Bacillus subtilis stressosome affect its basal output but not response to environmental signals. J. Bacteriol. 193:3588–3597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gaidenko TA, Kim TJ, Weigel AL, Brody MS, Price CW. 2006. The blue-light receptor YtvA acts in the environmental stress signaling pathway of Bacillus subtilis. J. Bacteriol. 188:6387–6395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hecker M, Pané-Farré J, Völker U. 2007. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 61:215–236 [DOI] [PubMed] [Google Scholar]
- 19. Igoshin OA, Brody MS, Price CW, Savageau MA. 2007. Distinctive topologies of partner-switching signaling networks correlate with their physiological roles. J. Mol. Biol. 369:1333–1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Janes BK, Stibitz S. 2006. Routine markerless gene replacement in Bacillus anthracis. Infect. Immun. 74:1949–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jones TA, Zou JY, Cowan SW, Kjeldgaard M. 1991. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr. A 47:110–119 [DOI] [PubMed] [Google Scholar]
- 22. Jurk M, Dorn M, Schmieder P. 2011. Blue flickers of hope: secondary structure, dynamics, and putative dimerization interface of the blue-light receptor YtvA from Bacillus subtilis. Biochemistry 50:8163–8171 [DOI] [PubMed] [Google Scholar]
- 23. Kang CM, Brody MS, Akbar S, Yang X, Price CW. 1996. Homologous pairs of regulatory proteins control activity of Bacillus subtilis transcription factor σB in response to environmental stress. J. Bacteriol. 178:3846–3853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kim TJ, Gaidenko TA, Price CW. 2004. A multicomponent protein complex mediates environmental stress signaling in Bacillus subtilis. J. Mol. Biol. 341:135–150 [DOI] [PubMed] [Google Scholar]
- 25. Kim TJ, Gaidenko TA, Price CW. 2004. In vivo phosphorylation of partner switching regulators correlates with stress transmission in the environmental signaling pathway of Bacillus subtilis. J. Bacteriol. 186:6124–6132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Losi A, Polverini E, Quest B, Gärtner W. 2002. First evidence for phototropin-related blue-light receptors in prokaryotes. Biophys. J. 82:2627–2634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lupas AN, Gruber M. 2005. The structure of α-helical coiled coils. Adv. Protein Chem. 70:37–78 [DOI] [PubMed] [Google Scholar]
- 28. Macek B, et al. 2007. The serine/threonine/tyrosine phosphoproteome of the model bacterium Bacillus subtilis. Mol. Cell. Proteomics 6:697–707 [DOI] [PubMed] [Google Scholar]
- 29. Marles-Wright J, et al. 2008. Molecular architecture of the “stressosome,” a signal integration and transduction hub. Science 322:92–96 [DOI] [PubMed] [Google Scholar]
- 30. Miller JH. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 31. Möglich A, Ayers RA, Moffat K. 2009. Design and signaling mechanism of light-regulated histidine kinases. J. Mol. Biol. 385:1433–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Möglich A, Ayers RA, Moffat K. 2009. Structure and signaling mechanism of Per-ARNT-Sim domains. Structure 17:1282–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Möglich A, Moffat K. 2007. Structural basis for light-dependent signaling in the dimeric LOV domain of the photosensor YtvA. J. Mol. Biol. 373:112–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Murray JW, Delumeau O, Lewis RJ. 2005. Structure of a nonheme globin in environmental stress signaling. Proc. Natl. Acad. Sci. U. S. A. 102:17320–17325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pané-Farré J, Lewis RJ, Stülke J. 2005. The RsbRST stress module in bacteria: a signalling system that may interact with different output modules. J. Mol. Microbiol. Biotechnol. 9:65–76 [DOI] [PubMed] [Google Scholar]
- 36. Parkinson JS. 2010. Signaling mechanisms of HAMP domains in chemoreceptors and sensor kinases. Annu. Rev. Microbiol. 64:101–122 [DOI] [PubMed] [Google Scholar]
- 37. Price CW. 2010. General stress response in Bacillus subtilis and related Gram positive bacteria, p 301–318 In Storz G, Hengge R. (ed), Bacterial stress responses, 2nd ed ASM Press, Washington, DC [Google Scholar]
- 38. Pruitt KD, Tatusova T, Maglott DR. 2007. NCBI reference sequences (RefSeq): a curated non-redundant sequence database of genomes, transcripts and proteins. Nucleic Acids Res. 35:D61–D65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quin MB, et al. 2012. The bacterial stressosome: a modular system that has been adapted to control secondary messenger signaling. Structure 20:350–363 [DOI] [PubMed] [Google Scholar]
- 40. Sambrook J, Fritsch EF, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 41. Stranzl GR, et al. 2011. Structural insights into inhibition of Bacillus anthracis sporulation by a novel class of non-heme globin sensor domains. J. Biol. Chem. 286:8448–8458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang X, Kang CM, Brody MS, Price CW. 1996. Opposing pairs of serine protein kinases and phosphatases transmit signals of environmental stress to activate a bacterial transcription factor. Genes Dev. 10:2265–2275 [DOI] [PubMed] [Google Scholar]
- 44. Zhang X-J, Matthews BW. 1995. EDPDB: a multifunctional tool for protein structure analysis. J. Appl. Crystallogr. 28:624–630 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.