Fig 5.
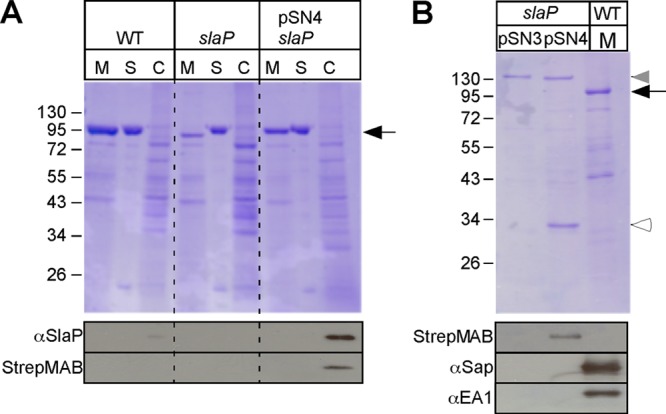
B. anthracis slaP mutants can be complemented by affinity-tagged slaP, which encodes a cytoplasmic protein. (A) Vegetative forms of B. anthracis Sterne (WT) and its slaP mutant without a plasmid or with pSN4, harboring slaP with a 3′ extension of its open reading frame specifying a Strep-tagged peptide, were grown to the mid-log phase. Cultures were centrifuged to separate the extracellular medium (M) from the bacterial sediment. Bacilli were extracted with hot urea to remove the S layer (S) and again centrifuged to sediment cells, which were subsequently broken in a bead beater (C). (Top) Proteins in all fractions were precipitated with TCA, washed in acetone, and analyzed with Coomassie-stained 10% SDS-PAGE gels. The position of Sap/EA1 is indicated by a black arrow. (Bottom) Samples were subjected to immunoblotting with a Strep-tag-specific monoclonal antibody, which identified SlaPStrep in B. anthracis slaP(pSN4) cells. (B) B. anthracis slaP(pSN3) and slaP(pSN4) cells were broken in a bead beater instrument, and cleared lysates were subjected to affinity chromatography on Strep-tactin Sepharose, eluted with desthiobiotin, and analyzed with Coomassie-stained SDS-PAGE gels. A medium (M) sample from B. anthracis Sterne cultures was added as a control. The black arrow identifies the position of Sap/EA1, and the white arrowhead identifies that of SlaPStrep. The gray arrowhead identifies the position of a B. anthracis protein in slaP(pSN3) and slaP(pSN4) cells that copurified during chromatography on Strep-tactin Sepharose but that did not react with the Strep-tag-specific monoclonal antibody (see below). Shown at the bottom are samples that were subjected to immunoblotting with a Strep-tag-specific monoclonal antibody or with rabbit antisera raised against purified Sap or EA1.
