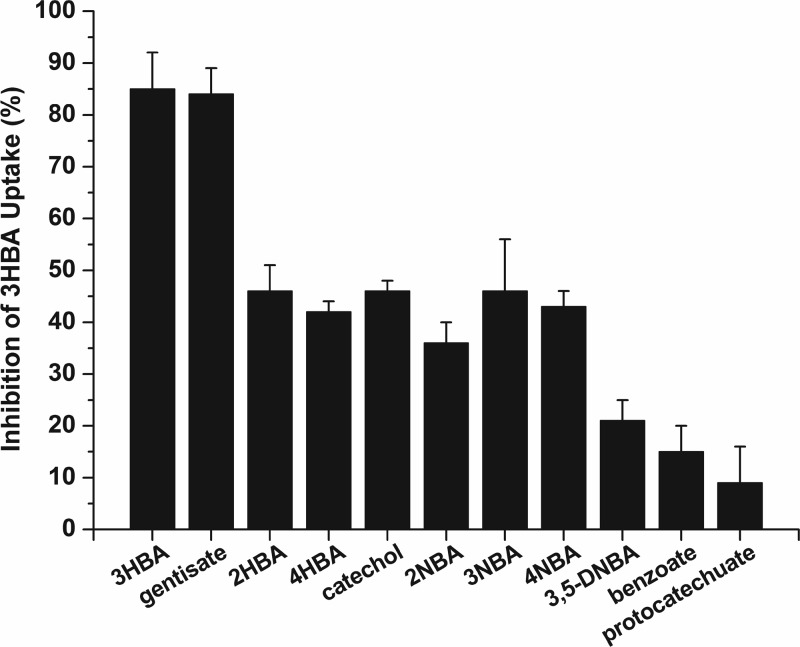
Fig 5.
Substrate inhibition of MhbT-mediated 3-hydroxybenzoate uptake in P. putida PaW340[pZWXY002]. The concentrations of 3-hydroxybenzoate and the competing substrate were 40 and 800 μM, respectively. The rate of accumulation of 3-hydroxybenzoate (without competing substrates) was 11.86 ± 0.54 nmol/min/mg of protein, which was set as 100% activity. Inhibition was determined by comparing the rates of 3-hydroxybenzoate uptake in the presence and absence of competing substrates. Values are the averages of data from three experiments ± standard deviations. 2HBA, 2-hydroxybenzoate; 3HBA, 3-hydroxybenzoate; 4HBA, 4-hydroxybenzoate; 2NBA, 2-nitrobenzoate; 3NBA, 3-nitrobenzoate; 4NBA, 4-nitrobenzoate; 3,5-DNBA, 3,5-dinitrobenzoate.