Abstract
This study examined the prospective longitudinal relationship between changes in depressive symptoms on alcohol and/or drug (i.e., substance) use among addiction treatment participants and whether group cognitive behavioral therapy for depression (GCBT-D) moderated the relationship. Using a quasi-experimental intent-to-treat design, 299 residential addiction treatment clients with depressive symptoms (Beck Depression Inventory-II (BDI-II) >17) were assigned to either usual care (n = 159) or usual care plus a 16-session GCBT-D intervention (n = 140). Two follow-up interviews were conducted, one three months after the baseline interview corresponding to the end of the intervention, and three months later. Parallel process growth modeling was used to examine changes in depressive symptoms and the associated changes in abstinence and negative consequences from substance use over time. Treatment group was included as a moderator of the association. Participants in the GCBT-D condition showed a greater increase in abstinence and greater decreases in depressive symptoms and negative consequences over time. There were significant interaction effects such that the associations between depressive symptoms, negative consequences, and abstinence changes were larger in the usual care condition compared to the GCBT-D condition. The results suggest that the intervention may be effective by attenuating the association between depressive symptoms and substance use outcomes. These findings contribute to the emerging literature on the prospective longitudinal associations between depressive symptoms and substance use changes by being the first to examine it among a sample receiving GCBT-D in an addiction treatment setting.
Keywords: co-occurring disorders, depression, substance use, cognitive behavioral therapy, treatment moderation
Introduction
Individuals with substance use disorders frequently suffer from depression, and co-occurring disorders (COD) are associated with poorer treatment outcomes, increased morbidity and mortality (Compton, Thomas, Stinson, & Grant, 2007; Hasin et al., 2002) and higher treatment costs (Clark, Samnaliev, & McGovern, 2009). An emerging literature suggests that providing cognitive behavioral therapy for depression within addiction treatment settings may improve both mood and substance use outcomes (Brown, Evans, Miller, Burgess, & Mueller, 1997; Brown et al., 2006; Watkins et al., 2011), however it is not well known how this treatment influences the relationship between depressive symptoms, substance use and negative consequences from use following treatment.
Depressed mood and substance use appear to be associated with one another, however the reported effects of depressed mood on substance use appear mixed (Suter, Strik, & Moggi, 2011; Tomlinson, Tate, Anderson, McCarthy, & Brown, 2006). Inconsistencies across studies in how (i.e., diagnosis versus symptoms) and when (i.e., pre-treatment, during or following treatment) depression and substance use are reported have made it difficult to draw conclusions about the temporal relationship between these two conditions (Glasner-Edwards et al., 2009; Gamble, Conner, Talbot, Yu, Tu & Connors, 2010). For example, some studies report that depressive symptoms tend to precede relapse among drinkers (Suter, Strik, & Moggi, 2011; Witkiewitz, Bowen, & Donovan, 2011), however other researchers have reported that a clinical diagnosis at the time of treatment entry, rather than symptom reporting, predicts return to drinking (Greenfield et al., 1998). A recent meta-analyses across 74 studies suggest that in general, depressive symptoms at the time of first measurement predict higher levels of alcohol related impairment at follow up (Conner, Pinquart, & Gamble, 2009). Recent meta-analyses of studies among cocaine (Conner, Pinquart, & Holbrook, 2008) and injection drug users (Conner, Pinquart, & Duberstein, 2007) demonstrate a small, concurrent relationship between depressive symptoms and substance use, but no evidence of a longitudinal relationship such that depressive symptoms influences future substance use. We are not aware of any research examining the prospective longitudinal associations between depressive symptoms, substance use and its related consequences among individuals receiving cognitive behavioral therapy for depression in addiction treatment settings.
It has been posited that cognitive behavioral therapy (CBT) for depression helps individuals to recognize negative affective states, re-conceptualize these states, and/or develop alternative coping strategies (Butler, Chapman, Forman, & Beck, 2006). In the context of substance use, CBT for depression may be effective in reducing continued substance use in a couple of ways. First it may reduce the occurrence of high levels of negative affect therefore reducing the likelihood for a maladaptive coping response (i.e., substance use). Second, it may reduce the association between negative affect and substance use by assisting individuals in developing alternative (i.e., non-substance use) responses when negative affective states are experienced. However, there is no empirical evidence that CBT for depression is effective for substance users by reducing the association between mood and substance use.
In order to enhance mental health and substance use outcomes among depressed substance users we developed and tested a group cognitive behavioral therapy for persistent depressive symptoms (GCBT-D; Hepner et al., 2011a) delivered as an adjunct to residential addiction treatment. Participants who reported elevated levels of depressive symptoms two-four weeks after treatment entry were eligible to participate in the study. We examined this group of patients because both major and minor depression significantly impair functioning and reduce health-related quality of life (Rapaport et al. 2002). Furthermore, given the difficulty of distinguishing between an independent depressive disorder in the context of substance use and a substance-induced disorder, we expect that, in typical addiction treatment settings, both groups would be offered treatment. By requiring that the depressive symptoms continue for at least two weeks after treatment admission, we excluded individuals whose impaired mood quickly improved after treatment entry or with sobriety. Among individuals with cocaine dependence, (Husband et al., 1996), opiate dependence (Strain, Stitzer, & Bigelow, 1991), and alcohol dependence (Brown & Schuckit, 1988), depressive symptoms decrease within the first 7–14 days after treatment admission and then remain stable over the next 4–8 weeks. Further, CBT is designed to prevent and reduce depressive symptoms in populations at high risk for major depression (Muñoz, 1993; Lewinsohn 1987), including individuals with minor depressive symptoms (Muñoz et al., 1995; Lewinsohn, Hoberman, & Clarke, 1989).
In this study, we examined: a) the associated changes in depressive symptoms, substance use and consequences from use during and following GCBT-D treatment; and b) whether GCBT-D influenced the association between depressive symptoms, substance use, and related consequences from use over time. We hypothesized that there would be a prospective, longitudinal association between depressive symptoms, substance use and consequences from use such that improvements in depressive symptoms would be associated with improvements on the substance use outcomes. We hypothesized that GCBT-D may improve outcomes by reducing the association between depressive symptoms, substance use and related consequences.
Methods
Setting
Study sites were four residential programs operated by Behavioral Health Services (BHS), one of the largest publicly-funded addiction treatment providers in Los Angeles County.
Design
We used an intent-to-treat quasi-experimental design in which cohorts of clients at each of the four study sites received either residential treatment as usual (UC) or residential treatment enhanced with the GCBT-D intervention. Assignment to treatment condition systematically alternated across the treatment sites over the course of the study. Participants assigned to the GCBT-D condition had other treatment-related group therapy commitments reduced accordingly so that clients in both study conditions received the same number of group treatment sessions per week. More information on the assignment schedule is available in Watkins et al. (2011).
Participants
Study recruitment began in August 2006 and ended in January 2009. During that period a total of 1,262 clients were screened for eligibility and a total of 299 clients experiencing persistent elevated depressive symptoms were enrolled (approximately 24% of the population). We defined persistent symptoms as symptoms that were measured on two separate occasions after at least two weeks of sobriety. Clients were first screened by residential staff using the Patient Health Questionnaire (PHQ-8; Spitzer, Kroenke & Williams, 1999) 14 days after entering treatment. Clients with a score of five or greater (corresponding to at least mild depression symptoms) were asked whether research staff could contact them. Fifty-nine percent of the clients screened at two weeks scored five or greater. Next, the research team conducted a second screening to determine eligibility; 9% of the sample refused the second screening or their contact information was lost, and 5% were discharged from the program before the second screening. Inclusion criteria assessed at the second screening included: 1) Beck Depression Inventory-II (BDI-II) scores > 17, indicative of moderate to severe depressive symptoms (Beck, Steer, & Brown, 1996; Buckley, Parker, & Heggie, 2001) and 2) the ability to speak and understand English. Exclusion criteria included self-reported bipolar disorder (Sloan, Kivlahan, & Saxon, 2000), schizophrenia (Wells, Sturm, & Burnam, 2001) and/or cognitive impairment (Dennis, White, Titus, & Unsicker, 2006). Clients on federal probation or parole were also excluded as permission from the Federal Parole Board was not obtained.
Study Conditions
GCBT-D Intervention Condition
The GCBT-D included 16 two-hour group sessions, divided into four modules: Thoughts, activities, people interactions, and substance use (Hepner et al., 2011a). The group was delivered twice a week and co-led by two addiction treatment counselors. Counselors that were employed in different settings were hired and trained to provide the treatment so to avoid contamination across study conditions. Enrollment into the group was semi-open, as new clients could enter the group at the beginning of each the four modules (i.e., every four sessions or every two weeks). Details on counselor training and fidelity are reported elsewhere (Hepner et al., 2011b; c); results indicated high adherence and competence to the treatment protocol.
Comparison Condition
The comparison condition consisted of treatment as usual (i.e., usual care or UC). Treatment across the sites was standardized. Clients experienced similar enrollment procedures and participated in individual substance use treatment counseling, group therapy, vocational skills training, AA/NA/CA meetings, recreational therapy, and family services. Residential staff were instructed to follow their usual mental health care procedures of referring clients with severe mental health conditions to a community mental health provider for evaluation and treatment as no onsite mental health treatment was available. Residential staff did not report receiving any formal mental health training before or during the study and did not receive any training in the GCBT-D during the study period.
Procedures
Treatment Assignment
Participants meeting study criteria were enrolled in one of two study conditions: UC or UC plus 8 weeks (16 sessions) of GCBT-D approximately three-four weeks after admission to residential treatment. Participants assigned to the intervention condition initiated the GCBT-D treatment within two weeks after study enrollment.
Data Collection
Following screening and consent, participants completed a semi-structured baseline interview conducted by trained field staff. Three months after the baseline interview, the first follow-up interview was administered by field staff. The timing of the first follow-up interview was after participants assigned to receive GCBT-D completed this treatment. Three months later (i.e., approximately six months after the baseline interview and three months after GCBT-D ended) a second follow-up interview was administered by field staff. We attempted to follow-up with all participants regardless of treatment status. Participants received $20 for completing the baseline and $30 for completing the three- and six-month follow-up interviews.
Measures
Baseline Measures
Demographic information including participant age, gender, race/ethnicity, marital status, education, and employment status was obtained in the baseline interview. Participants were also asked if they had been recently homeless, arrested or living in an institutionalized setting (e.g., jail, hospital other residential treatment setting). The Composite International Diagnostic Interview (CIDI; Walters, Kessler, Nelson, & Mroczek, 1998) was used to determine whether participants met the criteria for current major depression disorder and/or dysthymia. Participants were asked whether they were taking any medication for mental or emotional problems and if so, they were asked to provide the names of the medications so that participants could be classified as to whether they reported using an antidepressant or not. A modified version of an item from the Addiction Severity Index (McLellan, Carise, & Coyne, 2005) was used to assess problem substance. The Addiction Severity Evaluation Indices were used to assess past 12-month alcohol and drug use severity (Alterman et al., 1998). Participants were also asked whether they had received addiction treatment previously and whether they were currently attending self-help groups.
Outcomes
Depressive Symptoms
The Beck Depression Inventory-II (BDI-II) was used to assess depressive symptoms (Beck et al., 1996). The BDI-II is a 21-item scale that measures level of depressive symptoms within a previous two-week reference period and is widely used to evaluate the intensity of self-reported depression. It has been shown to be reliable for treatment-seeking substance users (Buckley et al., 2001), and in the current study the internal consistency of the BDI-II was (α = 0.92).
Substance Use
We calculated the percentage of days that participants reported being abstinent out of days available to use (i.e., not residing in an institutionalized setting, for example a hospital, treatment center or jail/prison) in the past 30 days using the TimeLine Followback method (Sobell & Sobell, 1995) for alcohol and the Addiction Severity Index (McLellan et al., 2005) for past 30-day illicit drug use. Both measures have been shown to be reliable and valid assessments of alcohol and drug use in similar populations to the one recruited for the current study (Alterman et al., 1998; Fals-Stewart, O'Farrell, Freitas, McFarlin, & Rutigliano, 2000; Sobell, Maisto, Sobell, & Cooper, 1979; Sobell & Sobell, 1978).
Negative Consequences from Use
Past 90-day consequences from use were assessed using the Shortened Inventory of Problems modified for alcohol and drug use (SIP-AD; Tonigan & Miller, 2002). The SIP-AD exhibited good internal consistency in the current study (α = 0.95) and has been shown to have adequate convergent and discriminant validity, and the ability to detect change over time (Blanchard, Morgenstern, Morgan, Lobouvie, & Bux, 2003).
Data Analytic Plan
Statistical models, described below, were estimated using Mplus version 6.1 (Muthén & Muthén, 2010). Given that participants were recruited from four different sites, all parameters were estimated using a weighted maximum likelihood function and all standard errors were adjusted using a sandwich estimator1 (the MLR estimator in Mplus). The MLR estimator provides the estimated variance-covariance matrix for the available data and therefore all available data were included in the models. Maximum likelihood is a preferred method for estimation when some data are missing, assuming the data are missing at random under the analytic model (Schafer & Graham, 2002). Attrition analyses revealed that individuals with any missing data across the three points (n = 63, 21%) had significantly higher BDI-II scores at 6-months (t (254) = −4.32, p < 0.001) in comparison to those with complete data on all measures (n = 236, 79%). However, there were no other significant differences on any study variables or measures collected in the GCBT-D study between those with missing data and those with complete data. Thus, using maximum likelihood estimation we assumed that data were missing at random given that BDI-II scores at 6-months were included in the model (i.e., the outcome was conditioned on BDI-II scores).
The current study utilized latent growth curve modeling and tests of moderation. Latent growth curve modeling was used to estimate the inter- and intra-individual change in BDI-II, PDA, and SIP-AD over time. The parameters derived from a latent growth model provide information about a construct’s average level (mean intercept) and average change over time (mean slope), as well as the individual variance around the intercept and slope. For the latent growth models, we first estimated a series of unconditional models (without covariates) to determine the form of growth (linear, quadratic, nonlinear). Model fit was evaluated by χ2 values, the Root Mean Square Error of Approximation (RMSEA) (Browne & Cudeck, 1993), and the Comparative Fit Index (CFI) (Bentler, 1990). Models with non-significant χ2, RMSEA less than 0.06 and CFI greater than 0.95 were considered a good fit to the observed data (Hu & Bentler, 1999). Models with RMSEA lower than 0.08 and CFI greater than 0.90 were considered an adequate fit to the observed data.
Moderation models were estimated using moderated regression (Aiken & West, 1991) within the context of a parallel process growth model as described by Cheong, MacKinnon and Khoo (2003) (Cheong, MacKinnon, & Khoo, 2003). We examined the change in BDI-II scores and change in substance use outcomes (PDA or consequences, estimated in separate models) from baseline to the 6-month follow-up. Treatment group was included as a predictor of both the change in BDI-II scores and change in substance use outcomes, as well as the random intercepts of BDI-II scores and substance use outcomes. In addition, changes in substance use outcomes were regressed on the interaction between treatment group and the change in BDI-II scores, which was estimated using a random slope that allowed for individual variation in the change in BDI-II scores at each level of the moderator (treatment group).
Results
Descriptive Statistics
Study participants were diverse in terms of sociodemographic and substance use characteristics (see Table 1). Most participants reported moderate to severe depressive symptoms and almost half met the criteria for a current depressive disorder. Many participants reported both alcohol and other illicit drug use (i.e., poly-substance use). The mean Addiction Severity Index evaluation scores were in line with typical levels of severity among clients entering addiction treatment settings (Alterman et al., 1998). Due to the baseline differences found between the GCBT-D and UC groups on self-help attendance and homelessness, we included these as covariates in the analyses. In addition, gender was incorporated as a covariate because of significant differences between males and females on the BDI-II at baseline (t (297) = 2.39, p = 0.02; Males Mean (SD) = 32.40 (9.08); Females Mean (SD) = 34.95 (9.31)).
Table 1.
Study sample baseline characteristics: Participants assigned to Group Cognitive Behavioral Therapy (GCBT-D) and Usual Care (UC)
| Characteristic | Overall (N=299) | GCBT-D (N=140) | UC (N=159) |
|---|---|---|---|
| Age (years), Mean (SD) | 36.2 (10.3) | 35.3 (10.1) | 37.0 (10.5) |
| Gender, % Male | 51.8 | 50.0 | 53.5 |
| Ethnicity, % | |||
| White | 33.8 | 37.1 | 30.8 |
| Hispanic | 30.1 | 27.9 | 32.1 |
| African American | 22.4 | 23.6 | 21.4 |
| Other | 13.7 | 11.4 | 15.7 |
| Education (years), Mean (SD) | 11.9 (2.0) | 11.8 (2.1) | 12.0 (2.0) |
| Married, % | 18.4 | 18.6 | 18.2 |
| Employed (full or part-time), % | 16.4 | 15.7 | 17.0 |
| Homeless (past 6 months), %* | 43.1 | 37.1 | 48.4 |
| Mental Health Measures | |||
| BDI-II score, Mean (SD) | 33.5 (9.2) | 32.7 (8.9) | 34.2 (9.5) |
| Taking psychiatric medication, % | 19.1 | 19.3 | 18.9 |
| CIDI, current depressive disorder, %a | 45.8 | 46.4 | 45.3 |
| Substance Use Measures | |||
| Negative consequences from use | 30.6 (11.8) | 29.5 (13.2) | 31.5 (10.3) |
| Lifetime | |||
| Ever received AOD treatment, % | 86.0 | 85.0 | 86.8 |
| Past 12 months | |||
| AUDIT-C, probable alcohol use disorder, % | 66.2 | 67.1 | 65.4 |
| ASI Alcohol Evaluation Index, Mean (SD) | 54.1 (9.8) | 54.0 (9.2) | 54.1 (10.4) |
| ASI Drug Evaluation Index, Mean (SD) | 47.5 (7.5) | 47.9 (7.8) | 47.2 (7.1) |
| Problem substance, % | |||
| Amphetamines | 36.8 | 40.0 | 34.0 |
| Cocaine | 20.4 | 21.4 | 19.5 |
| Alcohol | 15.4 | 12.9 | 17.6 |
| Heroin/Other Opiates/Analgesics Methadone | 12.4 | 12.9 | 12.0 |
| Alcohol and one or more drugs | 7.0 | 4.3 | 9.4 |
| More than one drug but no alcohol | 3.3 | 2.1 | 4.4 |
| Cannabis/Marijuana | 3.3 | 5.0 | 1.9 |
| Hallucinogens Sedatives/ Any other drug | 1.3 | 1.4 | 1.3 |
| Past 30 days | |||
| Attend self-help group, %** | 36.1% | 42.1% | 30.8% |
| Any arrest, % | 18.4 | 15.0 | 21.4 |
| Institutionalized for all 30 days, % | 15.7 | 14.3 | 17.0 |
| Percentage of days abstinent, Mean (SD)b | 42.1 (40.0) | 43.7 (40.8) | 40.7 (39.4) |
Note. BDI-II =Beck Depression Inventory-II; CIDI = Composite International Diagnostic Interview; AOD = alcohol or other drug; AUDIT-C = Alcohol Use Disorders Identification Test – Consumption; ASI = Addiction Severity Index.
Includes major depression and dysthymia.
From problem substance on days available to use.
denotes 0.05 < p < 0.10 between GCBT-D and UC groups.
denotes p < 0.05 between GCBT-D and UC groups.
GCBT-D participants attended a mean of 10.5 sessions (SD = 5.5) and 69% (n = 96) attended at least half of the 16 sessions. Two-hundred sixty (87.0%) and 256 clients (85.6%) completed three- and six-month post-baseline interviews. Response rates did not significantly differ between the study conditions at either wave (p = .55 and p = .77, respectively). Responders were not significantly different from non-responders at either wave with respect to baseline characteristics. At the time of the six-month follow-up, two-thirds of the sample had available days to use (i.e., were not institutionalized) and there were no difference across study condition (GCBT-D = 64% of the group had days available to use as compared to UC = 66%, p = .63). The length of stay in residential treatment did not differ between study conditions (GCBT-D Mean (SD) = 130.4 (72.3) days compared to UC Mean (SD) = 128.9 (68.8) days, p = .87). The percentage of clients reporting receiving external mental health treatment was also not statistically significant (i.e., 19% in GCBT group and 26% in UC group, p = .24) suggesting that participants received similar levels of mental health care outside of the study.
Outcome Variables
Descriptive statistics for selected outcome variables are reported in Table 2. Statistics are provided for all participants, and separately by treatment group. Independent samples t-tests were conducted to assess the significance of mean differences between groups. As seen in Table 2, individuals assigned to GCBT-D condition had significantly lower BDI-II scores, fewer negative consequences at three- and six-month follow-ups and higher percent of days abstinent at the six-month follow-up. The BDI-II and problem substance use results are reported elsewhere (Watkins et al., 2011). Correlation coefficients for all study variables, separated by treatment group, are provided in Table 3 with correlations for the GCBT-D participants below the diagonal and UC participants above the diagonal.
Table 2.
Descriptive Statistics, Mean (Standard Deviation), for Study Variables
| Variable | Total M (SD) | GCBT-D M (SD) | UC M (SD) | d |
|---|---|---|---|---|
| BDI-II baseline | 33.52 (9.23) | 32.72 (8.89) | 34.23 (9.49) | 0.16 |
| BDI-II 3-months | 18.59 (12.54) | 14.83 (11.23) | 21.82 (12.74)* | 0.58 |
| BDI-II 6-months | 15.45 (13.23) | 12.32 (11.90) | 18.16 (13.76)* | 0.46 |
| PDA baseline | 42.12 (40.02) | 43.66 (40.81) | 40.72 (39.40) | 0.07 |
| PDA 3-months | 85.90 (30.99) | 88.02(29.75) | 84.62 (31.97) | 0.11 |
| PDA 6-months | 85.83 (31.68) | 91.92 (22.89) | 80.29 (37.21)* | 0.39 |
| SIP-AD baseline | 30.59 (11.81) | 29.53 (13.23) | 31.54 (10.34) | 0.17 |
| SIP-AD 3-months | 8.80 (12.15) | 6.72 (10.08) | 10.60 (13.46)* | 0.33 |
| SIP-AD 6-months | 8.68 (12.89) | 6.34 (11.09) | 10.71 (13.99)* | 0.35 |
Note. n = 297; BDI-II = Beck Depression Inventory-II scores; PDA = Percent days abstinent; SIP-AD = Shortened Inventory of Problems.
Differences between groups based on independent samples t-test p < 0.05; d = Cohen’s d measure of effect size (small effect = 0.20; medium effect = 0.50; large effect = 0.80)
Table 3.
Correlations for Study Variables by Groups with GCBT-D Below Diagonal (n = 140) and UC Above Diagonal (n = 159)
| BDI-II baseline | BDI-II 3-months | BDI-II 6-months | PDA baseline | PDA 3-months | PDA 6-months | SIP-AD Baseline | SIP-AD 3-months | SIP-AD 6-months | |
|---|---|---|---|---|---|---|---|---|---|
| BDI-II baseline | -- | .54** | .29** | .10 | −.06 | −.07 | .14 | −.03 | −.06 |
| BDI-II 3-months | .16 | -- | .51** | .06 | −.49** | −.08 | .15 | .29** | .17 |
| BDI-II 6-months | .14 | .52** | -- | −.04 | −.35* | −.44** | .16 | .22* | .46** |
| PDA baseline | −.06 | .04 | −.05 | -- | −.08 | .26* | −.20* | −.05 | −.13 |
| PDA 3-months | −.06 | −.14 | −.06 | .47* | -- | .59** | −.33* | −.56** | −.54** |
| PDA 6-months | .05 | −.14 | −.24* | .40** | .88** | -- | −.17 | −.23* | −.70** |
| SIP-AD Baseline | .19* | −.04 | .03 | −.39** | −.16 | −.12 | -- | .29* | .14 |
| SIP-AD 3-months | .11 | .47** | .20* | .04 | −.54** | −.40** | .12 | -- | .48** |
| SIP-AD 6-months | .06 | .29** | .39** | −.16 | −.49* | −.66** | .15 | .46** | -- |
Note. Total n = 299; BDI-II = Beck Depression Inventory scores; PDA = Percent days abstinent; SIP-AD = Shortened Inventory of
Problems.
p < 0.05;
p < 0.01;
GCBT-D correlations below diagonal, UC correlations above diagonal.
Unconditional Growth Models
Latent growth curve modeling was used to examine the changes in BDI-II, SIP-AD, and PDA from baseline to a six-month follow-up. For all three measures the models with only a linear slope provided a poor fit to the data based on significant χ2 values, CFIs < 0.90 and RMSEAs > 0.08 across all three models. An inspection of the model estimated means and residuals suggested that a quadratic model might provide a better approximation of the observed data. Given only three time-points per measure, the latent growth models with linear and quadratic effects required an identifying restriction. With the restriction of the variance of the quadratic term to zero all three models were just-identified (e.g., χ2 (0) =0.00, CFIs = 1.00 and RMSEAs = 0.00).
The unconditional parallel process models of BDI-II with PDA and BDI-II with SIP-AD each provided a reasonable fit to the data (BDI-II with PDA: χ2 (7) = 22.10, p = .002; CFI = 0.94; RMSEA = 0.08 (90% CI of RMSEA: 0.05–0.13); BDI-II with SIP-AD: χ2 (7) = 16.36, p = .02; CFI = 0.95; RMSEA = 0.07 (90% CI of RMSEA: 0.02–0.11)). Coefficients for the fixed and random effects of the unconditional models are provided in Table 4. As seen in the table, the average BDI-II and SIP-AD scores significantly decreased over time, while PDA increased over time. The quadratic fixed effects suggested a significant deceleration with slight acceleration for BDI-II and SIP-AD scores; and a significant acceleration followed by deceleration for PDA. For both models the correlation between growth factors (shown as the first row of data in each section) were significant for linear slope (BDI-II with PDA r = −.90; BDI-II with SIP-AD r = .51). The correlation between the intercepts were only significant for BDI-II with SIP-AD scores (r = .25). Thus, the linear change in BDI-II and substance use outcomes was significantly associated over time, and the level of BDI-II was significantly associated with the level of consequences, but not with level of PDA.
Table 4.
Fixed and Random Effect Estimates for Unconditional Parallel Process Growth Models
| Fixed effects
|
Random effects
|
|||||
|---|---|---|---|---|---|---|
| Intercept | Linear | Quadratic | Intercept variance | Linear variance | Quadratic variance | |
| BDI-II with PDA | r =− 0.17 | r = −0.90** | ||||
| BDI-II scores | 33.52** | −19.69** | 5.39** | 67.48** | 47.58** | 0.00 |
| PDA | 5.10** | 6.12** | −2.14** | 0.92 | 0.28 | 0.00 |
|
| ||||||
| BDI-II with SIP-AD | r = 0.25** | r = 0.51** | ||||
| BDI-II scores | 33.52** | −19.75** | 5.41** | 63.98** | 38.80** | 0.00 |
| SIP-AD scores | 30.60** | −31.80** | 10.45** | 39.03* | 35.02* | 0.00 |
Note. n = 297; BDI-II = Beck Depression Inventory – II scores; PDA = Percent days abstinent; SIP-AD = Shortened Inventory of Problems.
p < 0.05;
p< 0.01.
Moderation Analyses
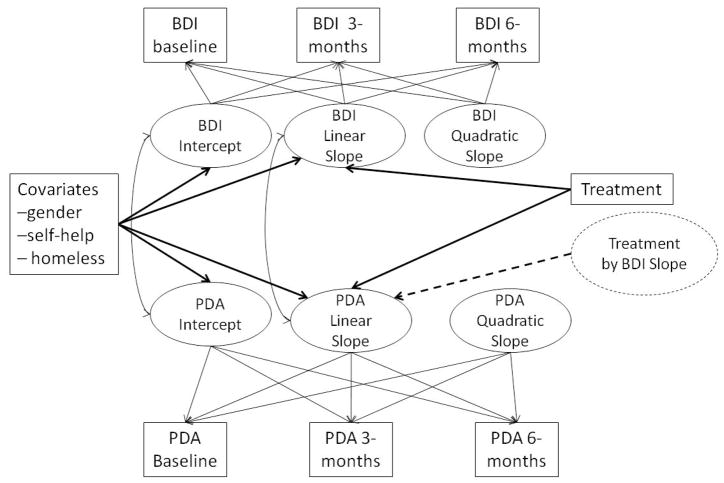
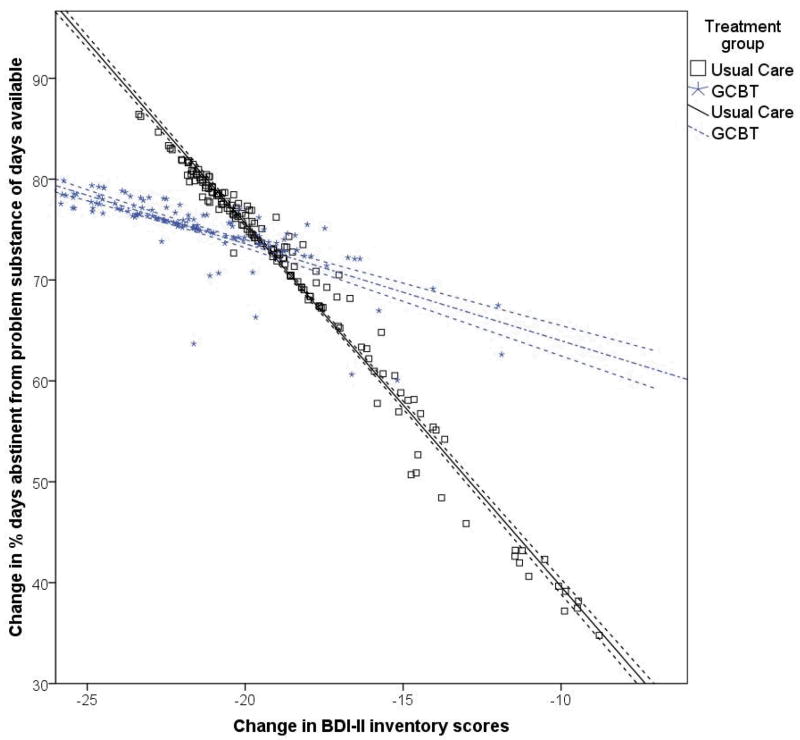
The second goal of the current study was to examine whether the association between the changes in BDI-II and substance use over time were moderated by treatment group. To accomplish this goal we estimated a parallel process growth model, shown in Figure 1, of the BDI-II growth process and substance use growth processes regressed on treatment condition. In addition, the linear slope of the substance use growth processes were regressed on the BDI-II linear slope, as well as the interaction between the linear slope of BDI-II with treatment condition. Results indicated a main effect of treatment condition in predicting BDI-II change (B (SE) = −3.20 (0.99), p = .001), PDA change (B (SE) = 6.14 (1.84), p = .001), and changes in SIP-AD (B (SE) = −8.97 (4.56), p = .04), with individuals assigned to GCBT-D reporting significantly greater decreases in BDI-II and SIP-AD scores, and significantly higher PDA over time. Consistent with the models described above, the change in BDI-II scores also predicted the change in PDA (B (SE) = −0.38 (0.09), p < .001) and SIP-AD scores (B (SE) = 1.08 (0.24), p < .001). The main effects of treatment and BDI-II scores were further qualified by a significant interaction between BDI-II slope and treatment group in the prediction of PDA slope (B (SE) = 0.32 (0.08), p < .001) and SIP-AD slope (B (SE) = −0.46 (0.21), p = .03). As seen in Figure 2, receiving the GCBT-D intervention attenuated the association between the changes in BDI-II and PDA, and between the changes in BDI-II and SIP-AD scores. In other words, the strength of the associations between the linear slopes of BDI-II and substance use outcomes was greater among the UC group and weaker among those in the GCBT-D study condition.
Figure 1.
Parallel process moderation model (residual variances not shown in figure, but were estimated in the model). Circles indicate latent variables and squares indicate observed variables. PDA = percent days abstinent; BDI-II = BDI-II inventory scores; Treatment = dummy coded comparison of Usual Care Control and GCBT-D groups.
Figure 2.
Association between the change in Beck Depression Inventory – II scores and the change in percent days abstinent from baseline to 6-months following the baseline assessment by treatment groups. Dashed lines indicate 95% confidence interval.
Discussion
The current study results demonstrate the prospective, longitudinal relationship between depressive symptoms on substance use and related consequences among a community-based residential addiction treatment sample experiencing elevated levels of depressive symptoms following treatment entry. Where prior studies have examined the relationship between both mild and moderate depressive symptom reported typically at baseline or at the end of treatment and a particular substance of abuse (e.g., Curran, Booth, Kirchner, & Deneke, 2007; Curran, Flynn, Kirchner, & Booth, 2000), this is the first study to examine this relationship among a heterogeneous mix of individuals receiving residential addiction treatment that report elevated symptoms as measured two to four weeks after treatment entry. This is important as sustained elevated symptom reporting after treatment entry may be an indicator of need for additional treatment provision. Moreover the results indicate that a GCBT-D intervention that targets elevated depressive symptoms is effective in decreasing the association between depressive symptoms and substance use over time, suggesting that one possible mechanism by which CBT may improve outcomes for co-morbid populations is by attenuating the relationship between negative affect and substance use. This is the first study that examined the prospective longitudinal association between these variables among a depressed addiction treatment sample receiving GCBT-D.
The current study results are consistent with other studies that have demonstrated a strong association between negative affective states and relapse (Hasin et al., 2002; Jaffe, Shoptaw, & Stein, 2007; Kodl et al., 2008; Tomlinson et al., 2006) and prior studies that have shown a dynamic (i.e., change over time) relationship between depressive symptoms and alcohol use among general (i.e., clinically depressed and non-depressed) outpatient treatment populations (Project MATCH) (Witkiewitz & Villarroel, 2009). In these prior studies, high negative affective states or increased negative affect over time increased the probability of drinking and conversely, heavier drinking predicted increased negative affect over time. Our study results are consistent with the findings that depressive symptoms may be related to future substance use and related consequences using a diverse (i.e., in terms of demographic and substance-related characteristics) treatment sample that typically comprise community based addiction treatment settings (Schaefer, Cronkite, & Hu, 2011). These findings emphasize that addressing depression in addiction treatment may improve outcomes.
These study findings are also consistent with studies examining other psychosocial approaches employed in addiction treatment, such as Mindfulness Based Relapse Prevention (MBRP) (Witkiewitz, Marlatt, & Walker, 2005), where mindfulness practices are taught to help increase client awareness of and reaction to negative emotional states. Research has shown that MBRP disrupts the relationship between depression and subsequent use through a reduction in the subjective experience of craving (i.e., an urge or desire to use) (Witkiewitz & Bowen, 2010). Additionally, researchers have demonstrated that a Coping with Cravings and Urges treatment module attenuated the relationship between negative mood and heavy drinking (Witkiewitz et al., 2011). The current study differs from these previous studies by specifically targeting individuals with persistent depressive symptoms. Also the current study demonstrated an association between depressive symptoms and negative consequences from substance use, an outcome not examined in previous studies. Substance use consequences appear to be a separate construct from substance use (Blanchard et al. 2002) and a relevant treatment outcome.
These study results are in line with findings from pharmacological studies on depression treatment among substance users. A recent meta-analyses concluded that when medication is effective in reducing depression, subsequent reductions in substance use are often exhibited (Nunes & Levin, 2004). It appears that participants who received cognitive behavioral therapy for depression in our study had a smaller association between negative affect and substance use following treatment than participants who did not receive the treatment. These findings underscore the importance of providing depression treatment for individuals with co-occurring depression and substance use disorders.
It is relevant to emphasize that the GCBT-D tested in this study was delivered in residential treatment. Many participants were still receiving addiction treatment at the three-month (70%) and six-month follow-up time points (35%). The lengthy stays in residential treatment (which were achieved, and not different, in both study conditions) decreased our ability to detect differences in substance use between the two groups and in the associations between substance use and depressive symptoms as many participants were residing in settings where use was prohibited. Given this, our study results suggest a fairly robust effect as associations were detectable even with the limited opportunity to use for many participants at the follow-up time points.
There are limitations to this research. First, our study used a quasi-experimental design. Although randomization would have been a stronger test of causality, we controlled for site and any baseline differences observed between the participants assigned to the two experimental conditions in our analyses. A second limitation was the use of self-reported substance and mental health measures that were not confirmed with physiological or collateral indicators. Participants may have under-reported their substance use or depressive symptoms although previous studies indicate the validity of these measures used with similar populations (Brown, Kranzler, & Del Boca, 1992; Rush et al, 2006; Sobell, Maisto, & Sobell, 1979; Sobell & Sobell, 1978; Weiss et al., 1998; Zimmerman, Coryell, Wilson & Corenthal, 1986). These data were collected by research staff not associated with the treatment program to reduce bias. Moreover, we do not think there would be a reason to observe systematic differences in reporting across study conditions that would have influenced the moderated treatment effect reported in this study. Third, our findings are specific to individuals with persistent elevated depressive symptoms in residential treatment. It is unknown whether the GCBT-D examined in this study may be effective in populations that exhibit milder forms of depressive symptoms or are treated in other (i.e., outpatient) settings. More work is needed to identify specific mechanisms of action responsible for attenuating the relationship between depressive symptoms, substance use and its associated consequences. For example, we only examined the cumulative effect of the GCBT-D intervention. We did not have the statistical power to evaluate the differential impact of each of the treatment modules, which would further clarify the specific GCBT-D component(s) and hypothesized mechanisms responsible for the moderating effect between depressive symptoms and the substance use outcomes. Also, future research is needed to better understand the timing of changes in depressive symptoms and use. We collected information about depressive symptoms in the past two weeks and substance use in the past 30-days at the baseline, three- and six-month interviews, limiting our ability to continuously monitor changes over time. Increasing the number of assessments may help to better illuminate the dynamic nature of depressive symptoms, substance use and related consequences. Examining session-specific effects and measurement frequency could lead to tailoring more effective and efficient delivery of GCBT-D in these settings. Finally, there were also limitations in our analytic approach. Missing data are often an issue in conducting longitudinal research. In the current study the retention rates exceeded 85%, response rates did not significantly differ between the conditions, and we used maximum likelihood estimation which is a preferred method for longitudinal analyses when some of the data are missing. Nonetheless, we cannot conclude that the results will generalize to those individuals who did not complete the follow-up assessments.
In summary, the study findings provide empirical evidence for the prospective longitudinal association between depressive symptoms on substance use and related consequences among a depressed residential addiction treatment population. Furthermore, the study demonstrated that cognitive behavioral therapy for depression may improve outcomes among depressed users by attenuating the relationship between depressive symptoms and substance use. Although more work is needed on the mechanisms underlying the treatment effects, the study results are consistent with pharmacology studies that indicate that depression treatment is associated with improved substance use outcomes, and with studies of individuals who experience negative affective states but who may not experience persistent depressive symptoms. The diversity among the study sample suggests that these results will generalize to many individuals receiving addiction treatment. Finally, this research supports the movement towards providing depression care in addiction treatment settings and empirical evidence on the efficacy of psychosocial treatments more broadly to decrease depressive symptoms, substance use and negative consequences from use.
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism to Katherine Watkins (grant no. 1R01AA014699-01A2); clinical trials identifier: NCT01191788. We thank Stephanie Woo (Pepperdine University) and Ricardo Muñoz (University of California San Francisco, San Francisco General Hospital, San Francisco State University) for their clinical expertise. We thank all of those at Behavioral Health Services, Inc., especially Jim Gilmore and Shirley Summers, for their administrative support along with the residential and outpatient treatment staff. A portion of the study results (i.e., main effects for depressive symptoms and problem substance use at six months) were reported previously in Watkins et al. (2011).
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/adb
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, London: Sage; 1991. [Google Scholar]
- Alterman AI, McDermott PA, Cook TD, Metzgera D, Rutherford MJ, Cacciola JS, et al. New scales to assess change in the addiction severity index for the opioid, cocaine, and alcohol dependent. Psychology of Addictive Behaviors. 1998;12(4):233–246. [Google Scholar]
- Beck A, Steer R, Brown G. BDI-II, Beck Depression Inventory: Manual. 2. Boston, MA: Harcourt Brace; 1996. [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychological Bulletin. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Blanchard KA, Morgenstern J, Morgan TJ, Lobouvie EW, Bux DA. Assessing consequences of substance use: Psychometric properties of the inventory of drug use consequences. Psychology of Addictive Behaviors. 2003;17(4):328–331. doi: 10.1037/0893-164X.17.4.328. [DOI] [PubMed] [Google Scholar]
- Brown J, Kranzler HR, Del Boca FK. Self-reports by alcohol and drug abuse inpatients: factors affecting reliability and validity. British Journal of Addiction. 1992;87(7):1013–1024. doi: 10.1111/j.1360-0443.1992.tb03118.x. [DOI] [PubMed] [Google Scholar]
- Brown RA, Evans DM, Miller IW, Burgess ES, Mueller TI. Cognitive-behavioral treatment for depression in alcoholism. Journal of Consulting and Clinical Psychology. 1997;65:715–26. doi: 10.1037//0022-006x.65.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Schuckit MA. Changes in depression among abstinent alcoholics. Journal of Studies on Alcohol. 1988;49:412–417. doi: 10.15288/jsa.1988.49.412. [DOI] [PubMed] [Google Scholar]
- Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Beverly Hills, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- Buckley TC, Parker JD, Heggie J. A psychometric evaluation of the BDI-II in treatment-seeking substance abusers. Journal of Substance Abuse Treatment. 2001;20(3):197–204. doi: 10.1016/s0740-5472(00)00169-0. [DOI] [PubMed] [Google Scholar]
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: A review of meta-analyses. Clinical Psychology Review. 2006;26(1):17–31. doi: 10.1016/j.cpr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Cheong J, MacKinnon DP, Khoo ST. Investigation of mediational processes using parallel process latent growth curve modeling. Structural Equation Modeling: A Multidisciplinary Journal. 2003;10(2):238–262. doi: 10.1207/S15328007SEM1002_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RE, Samnaliev M, McGovern MP. Impact of substance disorders on medical expenditures for Medicaid beneficiaries with behavioral health disorders. Psychiatric Services. 2009;60(1):35–42. doi: 10.1176/ps.2009.60.1.35. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2007;64(5):566–576. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- Conner KR, Pinquart M, Duberstein PR. Meta-analysis of depression and substance use and impairment among intravenous drug users (IDUs) Addiction. 2007;103:524–534. doi: 10.1111/j.1360-0443.2007.02118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner KR, Pinquart M, Gamble SA. Meta-analysis of depression and substance use among individuals with alcohol use disorders. Journal of Substance Abuse Treatment. 2009;37(2):127–137. doi: 10.1016/j.jsat.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conner KR, Pinquart M, Holbrook AP. Meta-analysis of depression and substance use and impairment among cocaine users. Drug and Alcohol Dependence. 2008;98:13–23. doi: 10.1016/j.drugalcdep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran GM, Booth BM, Kirchner JE, Deneke DE. Recognition and management of depression in a substance use disorder treatment population. The American Journal of Drug and Alcohol Abuse. 2007;33(4):563–569. doi: 10.1080/00952990701407496. [DOI] [PubMed] [Google Scholar]
- Curran GM, Flynn HA, Kirchner J, Booth BM. Depression after alcohol treatment as a risk factor for relapse among male veterans. Journal of Substance Abuse Treatment. 2000;19:259–265. doi: 10.1016/s0740-5472(00)00107-0. [DOI] [PubMed] [Google Scholar]
- Dennis ML, White MK, Titus JC, Unsicker JI. Short blessed scale exam. Global appraisal of individual needs: Trainer's training manual and resources (July 2006 version) Bloomington, IL: Chestnut Health Systems; 2006. [Google Scholar]
- Fals-Stewart W, O'Farrell TJ, Freitas TT, McFarlin SK, Rutigliano P. The timeline followback reports of psychoactive substance use by drug-abusing patients: Psychometric properties. Journal of Consulting and Clinical Psychology. 2000;68:134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- Gamble SA, Conner KR, Talbot NL, Yu Q, Tu XM, Connors GJ. Effects of pretreatment and posttreatment depressive symptoms on alcohol consumption following treatment in Project MATCH. Journal of Studies on Alcohol and Drugs. 2010;1:71–77. doi: 10.15288/jsad.2010.71.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasner-Edwards SV, Marinelli-Casey P, Hillhouse M, Ang A, Mooney LJ, Rawson RA, et al. Depression among methamphetamine users association with outcomes from the methamphetamine treatment project at 3-year follow-up. The Journal of Nervous and Mental Disease. 2009;197(4):225–231. doi: 10.1097/NMD.0b013e31819db6fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, Michael J. The effect of depression on return to drinking: a prospective study. Archives of General Psychiatry. 1998;55:259–265. doi: 10.1001/archpsyc.55.3.259. [DOI] [PubMed] [Google Scholar]
- Hasin D, Liu X, Nunes E, McCloud S, Samet S, Endicott J. Effects of major depression on remission and relapse of substance dependence. Archives of General Psychiatry. 2002;59(4):375–380. doi: 10.1001/archpsyc.59.4.375. [DOI] [PubMed] [Google Scholar]
- Hepner KA, Hunter SB, Paddock SM, Zhou AJ, Watkins KE. Training addiction counselors to implement CBT for depression. Administration and Policy in Mental Health and Mental Health Services Research. 2011c;38:313–323. doi: 10.1007/s10488-011-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepner KA, Miranda JM, Woo SM, Watkins KE, Lagomasino IT, Wiseman SH, et al. Building Recovery by Improving Goals, Habits, and Thoughts (BRIGHT): A group cognitive behavioral therapy for depression in clients with co-occurring alcohol and drug use problems -- group leader's manual. TR-977/1-NIAAA. Santa Monica, CA: RAND Corporation; 2011a. [Google Scholar]
- Hepner KA, Stern S, Paddock SM, Hunter SB, Osilla KC, Watkins KE. A fidelity coding guide for a group cognitive behavioral therapy for depression. TR- 980-NIDA/NIAAA. Santa Monica, CA: RAND Corporation; 2011b. [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Husband SD, Marlowe DB, Lamb RJ, Iguchi MY, Bux DA, Kirby KC, Platt JJ. Decline in self-reported dysphoria after treatment entry in inner-city cocaine addicts. Journal of Consulting and Clinical Psychology. 1996;64(1):221–224. doi: 10.1037//0022-006x.64.1.221. [DOI] [PubMed] [Google Scholar]
- Jaffe A, Shoptaw S, Stein JA. Depression ratings, reported sexual risk behaviors, and methamphetamine use: Latent growth curve models of positive change among gay and bisexual men in an outpatient treatment program. Experimental and Clinical Psychopharmacology. 2007;15(3):301–307. doi: 10.1037/1064-1297.15.3.301. [DOI] [PubMed] [Google Scholar]
- Kodl MM, Fu SS, Willenbring ML, Gravely A, Nelson DB, Joseph AM. The impact of depressive symptoms on alcohol and cigarette consumption following treatment for alcohol and nicotine dependence. Alcoholism: Clinical and Experimental Research. 2008;32(1):92–99. doi: 10.1111/j.1530-0277.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM. The coping with depression course. In: Munoz RF, editor. Depression Prevention: Research Directions. Washington, DC: Hemisphere; 1987. pp. 159–170. [Google Scholar]
- Lewinsohn PM, Hoberman HM, Clarke GN. The coping with depression course: Review and future directions. Canadian Journal of Behavioral Science. 1989;21:471–493. [Google Scholar]
- McLellan A, Carise D, Coyne T. Addiction Severity Index (ASI) 5. Philadelphia, PA: Treatment Research Institute; 2005. [Google Scholar]
- Muñoz RF. The prevention of depression: Current research and practice. Applied and Preventive Psychology. 1993;2:21–33. [Google Scholar]
- Muñoz RF, Ying YW, Bernal G, Perez-Stable EJ, Sorensen JL, Hargreaves WA, Miranda J, Miller LS. Prevention of depression with primary care patients: A randomized controlled trial. American Journal of Community Psychology. 1995;23:199–222. doi: 10.1007/BF02506936. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide (Version 6.1) Los Angeles, CA: Muthén & Muthén; 2010. [Google Scholar]
- Nunes EV, Levin FR. Treatment of depression in patients with alcohol or other drug dependence a meta-analysis. Journal of the American Medical Association. 2004;291(15):1887–1896. doi: 10.1001/jama.291.15.1887. [DOI] [PubMed] [Google Scholar]
- Rapaport MH, Judd LL, Schettler PJ, Yonkers KA, Thase ME, Kupfer DJ, Frank E, Plewes JM, Tollefson GD, Rush AJ. A descriptive analysis of minor depression. American Journal of Psychiatry. 2002;159(4):637–643. doi: 10.1176/appi.ajp.159.4.637. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Carmody TJ, Ibrahim HM, Trivedi MH, Biggs MM, Shores-Wilson K, Crismon ML, Toprac MG, Kashner TM. Comparison of self-report and clinician ratings on two inventories of depressive symptomatology. Psychiatric Services. 2006;57(6):829–837. doi: 10.1176/ps.2006.57.6.829. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychological Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- Schaefer JA, Cronkite RC, Hu KU. Differential relationships between continuity of care practices, engagement in continuing care, and abstinence among subgroups of patients with substance use and psychiatric disorders. Journal of Studies on Alcohol and Drugs. 2011;72(4):611–621. doi: 10.15288/jsad.2011.72.611. [DOI] [PubMed] [Google Scholar]
- Sloan K, Kivlahan D, Saxon A. Detecting bipolar disorder among treatment-seeking substance abusers. The American Journal of Drug and Alcohol Abuse. 2000;26(1):13–23. doi: 10.1081/ada-100100587. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Maisto SA, Sobell MB, Cooper AM. Reliability of alcohol abusers’ self-reports of drinking behavior. Behaviour Research and Therapy. 1979;17:157–160. doi: 10.1016/0005-7967(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Validity of self-reports in three populations of alcoholics. Journal of Consulting and Clinical Psychology. 1978;46(5):901–907. doi: 10.1037//0022-006x.46.5.901. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Alcohol timeline followback users' manual. Toronto: Addiction Research Foundation; 1995. [Google Scholar]
- Spitzer R, Kroenke K, Williams J. Validation and utility of a self-report version of PRIME-MD: The PHQ Primary Care Study. Journal of the American Medical Association. 1999;282:1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- Strain EC, Stitzer ML, Bigelow GE. Early treatment time course of depressive symptoms in opiate addicts. Journal of Nervous and Mental Disease. 1991;179:215–221. doi: 10.1097/00005053-199104000-00007. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. NSDUH Series H-36, HHS Publication No SMA 09–4434. Rockville, MD: Office of Applied Studies; 2009. Results from the 2008 National Survey on Drug Use and Health: National findings. [Google Scholar]
- Suter M, Strik W, Moggi F. Depressive symptoms as a predictor of alcohol relapse after residential treatment programs for alcohol use disorder. Journal of Substance Abuse Treatment. 2011;41(3):225–232. doi: 10.1016/j.jsat.2011.03.005. [DOI] [PubMed] [Google Scholar]
- Tomlinson KL, Tate SR, Anderson KG, McCarthy DM, Brown SA. An examination of self-medication and rebound effects: Psychiatric symptomatology before and after alcohol or drug relapse. Addictive Behaviors. 2006;31(3):461–474. doi: 10.1016/j.addbeh.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Tonigan JS, Miller WR. The Inventory of Drug Use Consequences (InDUC): Test-retest stability and sensitivity to detect change. Psychology of Addictive Behaviors. 2002;16(2):165–168. [PubMed] [Google Scholar]
- Walters E, Kessler R, Nelson C, Mroczek D. Composite International Diagnostic Interview (CIDI) 2.1. Geneva, Switzerland: World Health Organization (WHO); 1998. [Google Scholar]
- Watkins KE, Hunter SB, Hepner KA, Paddock SM, de la Cruz E, Zhou AJ, et al. An effectiveness trial of group cognitive behavioral therapy for patients with persistent depressive symptoms in substance abuse treatment. Archives of General Psychiatry. 2011;68(6):577–584. doi: 10.1001/archgenpsychiatry.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Najavits LM, Greenfield SF, Soto JA, Shaw SR, Wyner D. Validity of substance use self-reports in dually diagnosed outpatients. American Journal of Psychiatry. 1998;155(1):127–128. doi: 10.1176/ajp.155.1.127. [DOI] [PubMed] [Google Scholar]
- Wells K, Sturm R, Burnam M. Healthcare for Communities HCC1 Questionnaire. Santa Monica, CA: RAND Corporation; 2001. Healthcare for Communities (HCC) Psychoticism screener. [Google Scholar]
- Witkiewitz K, Bowen S. Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. Journal of Consulting and Clinical Psychology. 2010;78(3):362–374. doi: 10.1037/a0019172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, Donovan DM. Moderating effects of a craving intervention on the relation between negative mood and heavy drinking following treatment for alcohol dependence. Journal of Consulting and Clinical Psychology. 2011;79(1):54–63. doi: 10.1037/a0022282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Marlatt GA, Walker D. Mindfulness-based relapse prevention for alcohol and substance use disorders. Journal of Cognitive Psychotherapy: An International Quarterly. 2005;19(3):211–228. [Google Scholar]
- Witkiewitz K, Villarroel N. Dynamic association between negative affect and alcohol lapses following treatment. Journal of Consulting and Clinical Psychology. 2009;77(4):633–644. doi: 10.1037/a0015647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman M, Coryell W, Wilson ST, Corenthal C. Evaluation of symptoms of major depressive disorder. Self-report vs. clinician ratings. Journal of Nervous and Mental Disease. 1986;174(3):150–153. doi: 10.1097/00005053-198603000-00004. [DOI] [PubMed] [Google Scholar]