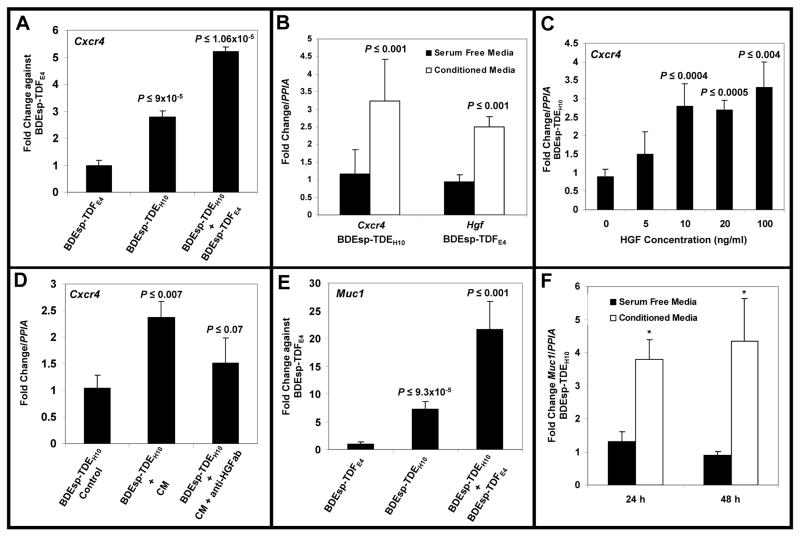
Figure 7.
(A) TaqMan® QRT-PCR data demonstrating that co-culturing of rat BDEsp-TDEH10 cholangiocarcinoma cells in rat tail type I collagen gel matrix in the presence of BDEsp-TDFE4 CAFs grown to confluence on type I collagen-coated plastic significantly induced Cxcr4 gene expression in the cholangiocarcinoma cells over monoculture cell values. (B). QRT-PCR data showing conditioned medium from cultured BDEsp-TDFE4 cells to significantly up-regulate Cxcr4 gene expression in BDEsp-TDEH10 cholangiocarcinoma cells cultured alone on type I collagen-coated plastic, whereas conditioned medium from BDEsp-TDE H10 cells significantly induced Hgf gene expression in BDEsp-TDFE4 cells cultured alone under identical conditions. (C) QRT-PCR data showing a significant concentration-dependent increase in Cxcr4 gene expression in cultured BDEsp-TDEH10 cells following exposure over a 48-hour treatment period to recombinant human HGF. (D) QRT-PCR data showing the effect of conditioned medium from cultured BDEsp-TDFE4 CAFs on up-regulating Cxcr4 gene expression in cultured BDEsp-TDEH10 cholangiocarcinoma cells is partially blocked by the addition of neutralizing HGF antibody (anti-HGFab). (E) QRT-PCR data demonstrating that co-culturing of BDEsp-TDEH10 cholangiocarcinoma cells in the presence of BDEsp-TDFE4 CAFs under identical conditions as in A also produced a significant up-regulation of Muc1 gene expression in the cholangiocarcinoma cells over monoculture cell values. (F) Similar to B, conditioned medium from cultured BDEsp-TDFE4 CAFs induced a significant increase in Muc1 gene expression in cultured BDEsp-TDEH10 cholangiocarcinoma cells. PPIA (cyclophilin A) was used as the housekeeping gene to normalize Cxcr4, Hgf and Muc1 gene expression. Comparable QRT-PCR results were also obtained when normalized with Gapdh. Each value represents the mean ± SD, obtained from determinations made on a minimum of six cultures per experimental condition.