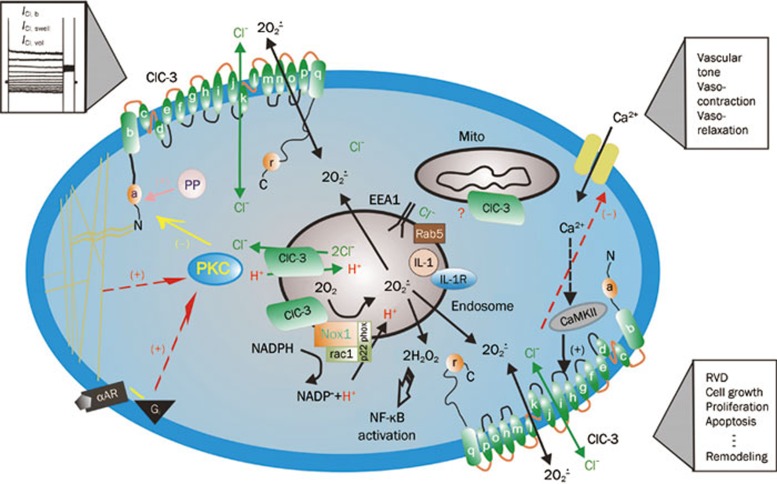
Figure 1.
Schematic representation of regulation and function of ClC-3 Cl− channels in cardiac myocytes and vascular smooth muscle cells. ClC-3, a member of voltage-gated ClC Cl− channel family, encodes Cl− channels in cardiac myocytes and vascular smooth muscle cells that are volume regulated (ICl, vol) and can be activated by cell swelling (ICl, swell) induced by exposure to hypotonic extracellular solutions or possibly membrane stretch. ICl, b is a basally activated ClC-3 Cl− current. Membrane topology model (α-helices a–r) for ClC-3 is modified from Dutzler et al100. ClC-3 proteins are expressed on both sarcolemmal membrane and intracellular organelles including mitochondria (mito) and endosomes. The proposed model of endosome ion flux and function of Nox1 and ClC-3 in the signaling endosome is modified from Miller Jr et al101. Binding of IL-1β or TNF-α to the cell membrane initiates endocytosis and formation of an early endosome (EEA1 and Rab5), which also contains NADPH oxidase subunits Nox1 and p22phox, in addition to ClC-3. Nox1 is electrogenic, moving electrons from intracellular NADPH through a redox chain within the enzyme into the endosome to reduce oxygen to superoxide. ClC-3 functions as a chloride-proton exchanger, required for charge neutralization of the electron flow generated by Nox1. The ROS generated by Nox1 result in NF-κB activation. Both ClC-3 and Nox1 are necessary for generation of endosomal ROS and subsequent NF-κB activation by IL-1β or TNF-α in VSMCs. PKC, protein kinase C; PP, serine-threonine protein phosphatases; α-AR, α-adrenergic receptor; Gi, heterodimeric inhibitory G protein; Nox: NADPH oxidase; CaMKII: Ca2+/calmodulin-dependent protein kinase II; (+) stimulation; (–) inhibition.