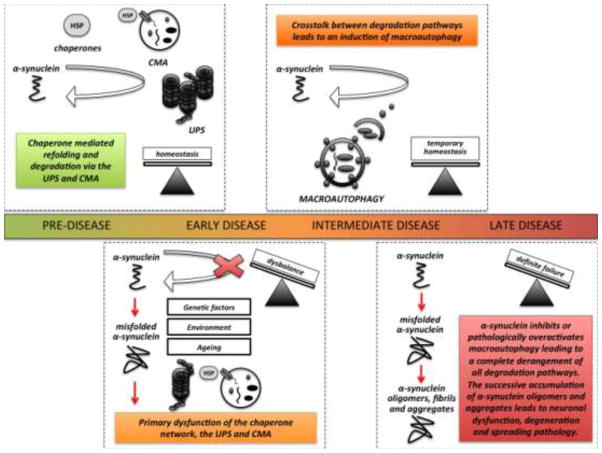
Fig. 3.
The successive failure of protein degradation pathways might provide a key step in the pathological cascade that leads to α-synuclein pathology, neuronal dysfunction and spreading neurodegeneration. The regular turnover of unmodified α-synuclein is largely mediated via both the ubiquitin-proteasome system (UPS) and chaperone-mediated autophagy (CMA). In the early disease stage, primary dysfunction of protein degradation, caused and influenced by genetic, environmental and age-related factors, may lead to excess levels of α-synuclein and the formation of pathologically modified species that overwhelm the capacity of chaperones, the UPS and CMA. Accumulating α-synuclein leads to further and continuous impairment of these pathways therefore eventually creating a vicious cycle. In the next disease stage, crosstalk between degradation pathways drives the induction of macroautophagy and temporary protein homeostasis is achieved before this balance finally tilts. Entering the late disease stage, α-synuclein inhibits or disproportionally overactivates dysfunctional macroautophagy and lysosomal degradation, leading to a complete derangement of all protein degradation pathways. Uncontrolled accumulation of α-synuclein produces oligomeric species that cause neuronal dysfunction and degeneration. At the same time, excess levels of α-synuclein and impaired degradation trigger α-synuclein secretion, thus promoting seeding or protein templating and therefore potentially contributing to a spread of the pathology. CMA (chaperone mediated autophagy); HSP (heat shock proteins); UPS (ubiquitin-proteasome-system)