Figure 2.
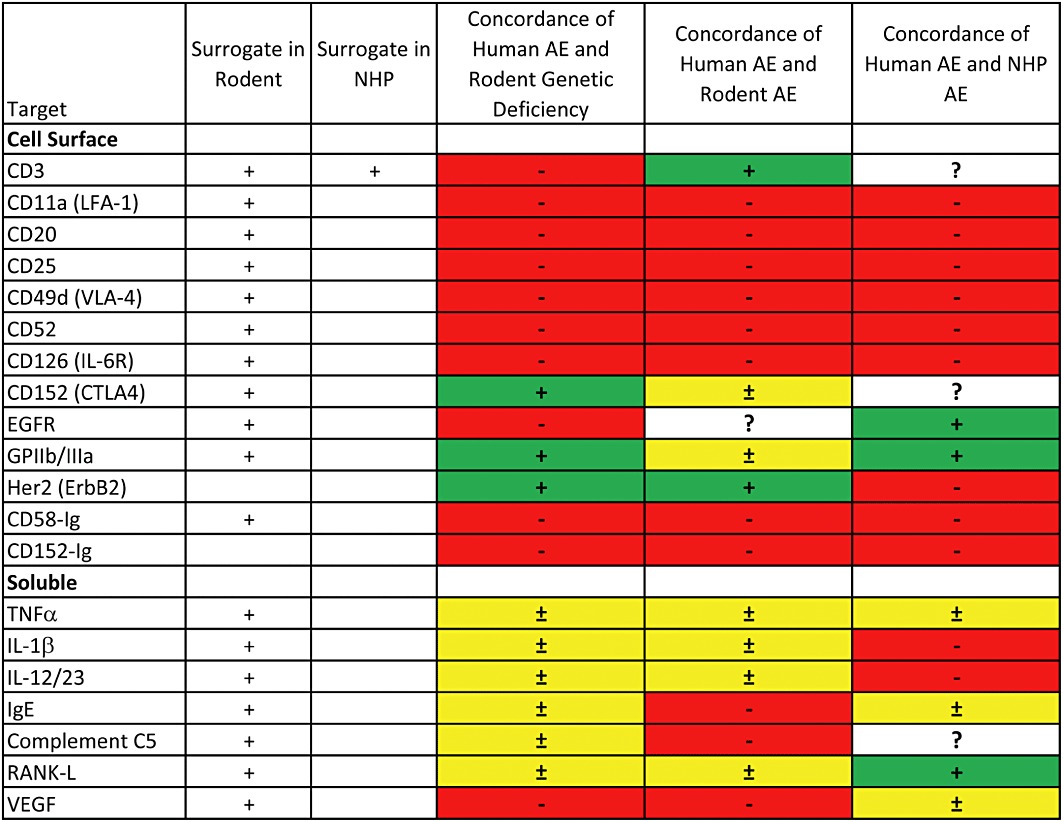
Summary data on concordance of human adverse effects (AEs) with genetically deficient mice, mice receiving a surrogate construct* or cynomolgus monkeys receiving the human biopharmaceutical. Green indicates an accurate reflection of the major effects of the biopharmaceutical in humans. Yellow indicates that some of the major effects in humans are not reflected in the preclinical data. Red indicates that major effects in humans are not reflected in the preclinical data. *In the cases of sCD58 (alefacept) and sCD152 (abatacept), the human biopharmaceutical was tested in mice. In the case of CD3, a surrogate was tested in NHP.
