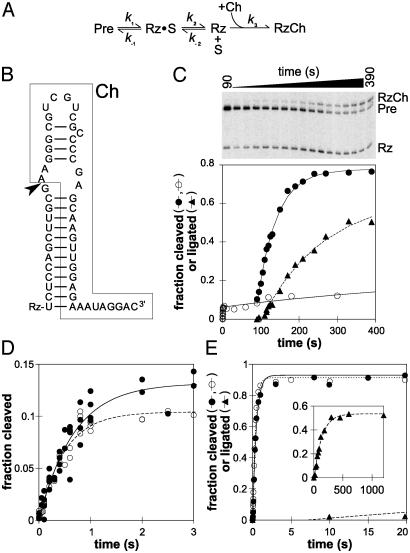
Fig. 3.
The natural cleavage site loop favors ligation. (A) Minimal kinetic scheme for self-cleavage in the presence of Ch. Cleavage of Pre yields 5′ and 3′ products designated Rz and S, respectively (see text). (B) Sequence and secondary structure of Ch (boxed) bound to Rz; only the nucleotides in Rz that pair with Ch are indicated. The arrowhead marks the site of ligation. (C) Time course of a RS19ΔL Ch experiment. The fraction of Pre cleaved in the absence of Ch (○) or after addition of Ch (to 1 μM) at t = 90 s (•); the fraction of Pre converted to RzCh, i.e., fraction ligated (▴); the smaller cleavage product, S, has run off the gel. (D) The burst phase of RS19ΔL cleavage in the absence of Ch (○) or with Ch added from the start of the experiment (•). Data from multiple experiments are shown. (E) Cleavage of RS19 in the absence (○) or presence (•)ofCh; ▴, fraction ligated. (Inset) Longer time course to show the appearance of RzCh; x axis, time (s); y axis, fraction ligated.