Figure 3.
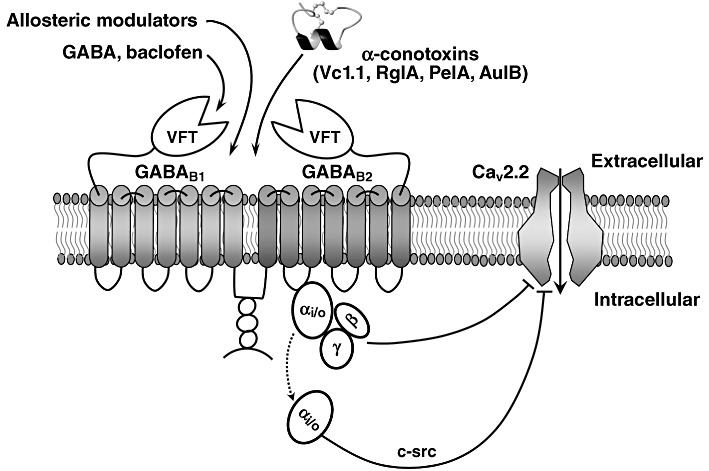
GABAB receptor activation by analgesic α-conotoxins. The highly conserved structural scaffold of the α-conotoxins consists of two disulfide bonds that stabilize a central helical region. GABAB receptor is a heterodimer with two almost identical subunits that are both required for a functional receptor. The GABAB1 subunit is involved in ligand binding and the GABAB2 subunit interacts with the G-protein. The natural ligand of the receptor, GABA, binds to a cleft within the large N-terminal ‘Venus fly-trap (VFT)’ domain of the GABAB1 subunit, triggering a conformational change in the receptor that facilitates interaction with the G-protein by the GABAB2 subunit. GPCR activation leads to dissociation of Gα from Gβγ subunits. A single GPCR can couple to either one or more families of Gα proteins, which activate several downstream effectors (Tedford and Zamponi, 2006). Upon ligand binding, Gβγ subunits function as a dimer to interact with many signalling molecules and with ion channels. The schematically shown pore-forming α1-B subunit of the N-type (Cav2.2) VGCC consists of IV homologous domains linked by cytoplasmic loops (referred to as I-II, II-III and III-IV linkers) and cytoplasmic N- and C-terminal regions.
