Abstract
Background.
The implementation of mass vaccinations against hepatitis B virus (HBV) has significantly reduced the prevalence of HBsAg-positive subjects. At the same time, the prevalence of the other markers of infection has decreased, but there has been an increase in the percentage of subjects with markers of a successful vaccination. It has been suggested that increasing immigration from countries in which this virus is highly endemic is changing the epidemiology of HBV infection. The aim of this study was to assess the prevalence of the serological markers of HBV in Italian and non-Italian HBsAg-negative subjects.
Materials and methods.
In the years 2007–2008, 8,018 samples from HBsAg-negative subjects (7,521 Italians and 497 non-Italians) were received for detection of anti-HBs and/or anti-HBc. The findings in the 1,358 samples from candidate blood donors were compared with those obtained in 1991 and 1999.
Results.
The rate of anti-HBc positivity was 18.3% in the Italian samples and 32.8% in the non-Italian samples; the corresponding percentages of anti-HBs/anti-HBc positive samples (indicating past infection), anti-HBs positive only samples (vaccination) and anti-HBc positive only were, 11.3% vs. 22.5%, 25.8% vs. 17.2%, and 6.9% vs. 9.9% in Italians and non-Italians, respectively. The differences were more marked when stratified by age. In relation to candidate blood donors, simultaneous positivity for anti-HBs and anti-HBc decreased from 11.0% in 1991 to 8.1% in 1999 and 3.9% in 2007–2008, whereas isolated anti-HBs positivity increased from 2.2% in 1991 to 21.4% in 1999 and 42.9% in 2007–2008.
Conclusions.
The frequency of markers of past infection among Italians has decreased over time as a result of mass vaccination and is significantly lower than that observed in non-Italians. The increasing number of immigrants from countries in which HBV is highly endemic is changing the epidemiology of HBV infection in Italy.
Keywords: HBV vaccination, epidemiology, HBV markers
Introduction
Hepatitis B virus (HBV) is ubiquitous, but the prevalence of infection varies from region to region. In countries in which HBV is highly endemic (Africa and parts of Asia), the seroprevalence of hepatitis B surface antigen (HBsAg) is ≥8%, whereas it ranges from 2% to 7% in southern Europe, the Middle East and South Asia, and is <2% in western Europe and North and South America (except for some Amazonian regions in Brazil and Peru)1. The median seroprevalence of antibodies to hepatitis B core antigen (anti-HBc) follows patterns similar to that of HBsAg, with reported values of 50.2%–56.2% for the African Region, 29.7%–41% for the Western Pacific Region, 25%–32.4% for the South-East Asia Region, 18.7%–31% for the Eastern Mediterranean Region, 7%–37.8% for the European Region and 11.6%–15% for the American Region2. The pattern of isolated anti-HBc is also related to the prevalence of HBV infection, ranging among blood donors from 0.4 to 1.7% in areas of low HBV prevalence3–8 to 10–20% in high endemic areas9,10. The implementation of mass vaccination programmes recommended by the World Health Organisation since 1991 has greatly decreased the incidence of HBV infection among children and adolescents11, and the prevalence of HBsAg positivity has simultaneously decreased12,13.
In Italy a selective vaccination of people at risk (intravenous drug users, male homosexuals, subjects with multiple sexual partners, the partners of HBsAg-positive patients, haemophiliacs, and infants of HBsAg-positive mothers) began on a regional basis in 1983. In 1991 (Law 165 of 27 May 1991), vaccination was made compulsory for all children aged 12 years and infants in the first year of life14,15. In a national survey in 1993–94 the overall vaccination coverage was 93.6%, with differences between northern (97.9%), central (96.6%) and southern Italy (65.1%)16. By the end of 2007, more than 15 million children had been vaccinated against hepatitis B17.
As a result of all these factors both the incidence of acute hepatitis and the prevalence of HBsAg carriers have decreased substantially18,19. From a prevalence of 3.5%17 in 1970–1980s, the prevalence of HBsAg carriers is now estimated to be less than 2%20. In Lombardy (a region of northern Italy in which our hospital is located) the prevalence among blood donors decreased from 2.7% in the 1980s21 to 0.14% in 200922. Other reasons that helped to change the epidemiology of HBV in Italy over the last 30–40 years include improved socio-economic conditions, better hygiene (screening of blood units and blood components, disinfection and sterilisation of medical and surgical instruments, introduction and use of disposable equipment, environmental education campaigns and better hospital care), smaller families, and educational campaigns against human immunodeficiency virus which encouraged the use of condoms and disposable syringes for intravenous drug users23.
The decrease in HBV infection was also evaluated on the basis of the prevalence of other serological markers, particularly anti-HBc, combined with antibodies to hepatitis B surface antigen (anti-HBs) indicating a past infection or isolated finding (with negative anti-HBs)24. For example, studies conducted in a hyperendemic area of southern Italy showed a decrease in anti-HBc levels from 66.9% in 1980 to 7.6% in 200625. Among national service recruits, the level decreased from 16.8% in 1981 to less than 1% in 200117,26 and among blood donors it decreased from 30% in the 1970s and 1980s27 to 5% in 200028. Since the introduction of vaccination, a population of young adults aged less than 30 years has emerged with almost no markers of past HBV infection17.
However, despite the decreased frequency of the markers of a current or previous infection among native Italians, a previously unforeseen change in epidemiology seems to be emerging as a result of increasing numbers of immigrants from countries in which HBV is highly endemic29.
The aim of this retrospective study was to evaluate the prevalence of the markers of past infection and of response to vaccination in Italian and non-Italian subjects living in the catchment area of the hospital of Legnano (an urban town in northern Italy) in 2007–2008.
Materials and methods
The hospital of Legnano is located in an urban area of northern Italy near Milan, and has a catchment area that includes approximately 250,000 people. It has general and specialised medical and surgical departments, and points for collecting blood samples from outpatients referred for a laboratory check-up by their general practitioners. It has a Blood Transfusion Centre that tests samples from blood donors and a Microbiology Unit that tests samples from in-patients and out-patients.
Between 1 January 2007 and 31 December 2008, samples from 22,758 subjects were received to be assayed for the presence of HBsAg, of which 22,270 (97.9%) were HBsAg-negative (Hepanostika HBsAg Ultra and Hepanostika HBsAg Ultra Confirmatory, BioMérieux, Boxtel, The Netherlands).
An assay for at least one other marker (enzyme immunoassay: anti-HBs [ETI-AB-AUK-3, DiaSorin, Saluggia, Italy] and/or anti-HBc total [HBc ELISA Test System, Ortho-Clinical Diagnostics, Raritan, USA]) had been requested for 8,018 of these subjects: 3,332 (41.6%) in-patients (1,962 men and 1,370 women; mean age 61.9 years, range 1–109 years), 3,328 (41.5%) out-patients referred by their general practitioners for laboratory check-ups (1,431 men and 1,897 women; mean age 46.3 years, range 1–104 years), and 1,358 (16.9%) candidate blood donors whose suitability for a first blood donation was being investigated (750 men and 608 women; mean age 33.1 years, range 18–62 years). Seven thousand five hundred and twenty-one of these subjects were Italians (93.8%) and 497 (6.2%) were non-Italians: 164 came from Eastern Europe, 130 from Latin America, 58 from the Middle East and the Maghreb, 56 from South-Equatorial Africa, 40 from the Indian sub-continent, 33 from Western Europe and North America and 16 from China and the Far East. The non-blood donor patients are representative of the local population as most of them were born and are residents in our catchment area.
The anti-HBc positive samples (but HBsAg/anti-HBs negative) were also tested with an enzyme linked fluorescent assay (VIDAS Anti-HBc Total, BioMérieux, Boxtel, The Netherlands).
The data relating to the candidate blood donors were compared with those obtained from similar groups in 1991 and 199930. The findings were statistically analysed using the χ2 test and Fisher’s exact test.
Results
A search for anti-HBs had been requested for 7,523 of the 8,018 subjects, and was positive in 2,654 (35.3%; 95% confidence interval [CI]: 34.22–36.38): in 2,487 of 7,058 Italian subjects (35.2%; 95% CI: 34.09–36.31) and in 167 of 465 non-Italian subjects (35.9%; 95% CI: 31.54–40.26). The difference in the prevalence between the two groups was not statistically significant. A search for total anti-HBc had been requested in 5,371 cases, and was positive in 1,027 (19.1%; 95% CI: 18.05–20.15): in 931 of 5,078 Italian subjects (18.3%; 95% CI: 17.24–19.36) and in 96 of 293 non-Italian subjects (32.8%; 95% CI: 27.42–38.18). The difference in the prevalence between the two groups was statistically significant (p<0.01).
A simultaneous search for anti-HBs and anti-HBc had been requested for 4,873 patients. Five hundred and seventy-eight subjects (11.9%; 95% CI: 10.99–12.81) were anti-HBs positive/anti-HBc positive, 1,235 (25.3%; 95%CI: 24.08–26.52) anti-HBs positive/anti-HBc negative and 345 anti-HBs negative/anti-HBc positive with results confirmed for 344 (7.1%; 95% CI: 6.38–7.82) by enzyme linked fluorescent assay.
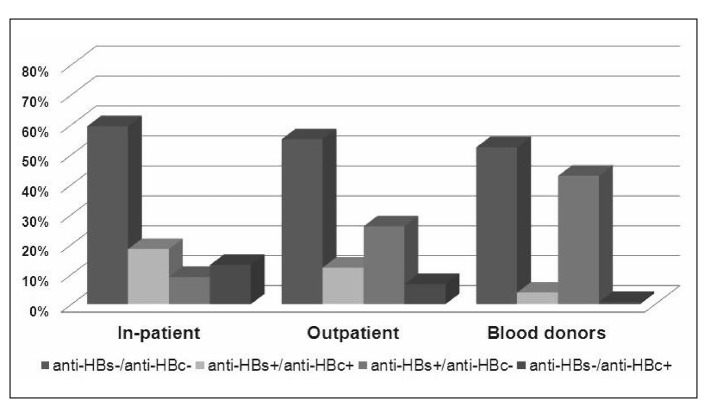
In 1,543 in-patients, 1,972 out-patients and 1,358 candidate blood donors the prevalence of the anti-HBs positive/anti-HBc positive pattern was respectively 18.4% (85% CI: 16.47–20.33), 12.2% (95% CI: 10.76–13.64) and 3.9% (95% CI: 2.87–4.93). The prevalence of the anti-HBs positive/anti-HBc negative pattern was 9.1% (95% CI: 7.66–10.54), 26.0% (95% CI: 24.06–27.94) and 42.9% (95% CI: 40.27–45.53), respectively, while that of the anti-HBs negative/anti-HBc positive pattern was 13.1% (95% CI: 11.42–14.78), 6.7% (95% CI: 5.60–7.80) and 0.7% (95% CI: 0.26–1.14), respectively. The differences in the prevalence for these patterns in the three groups were all statistically significant (p<0.01) (Figure 1).
Figure 1.
HBV serological patterns in HBsAg-negative in-patients, outpatients and candidate blood donors (2007–2008).
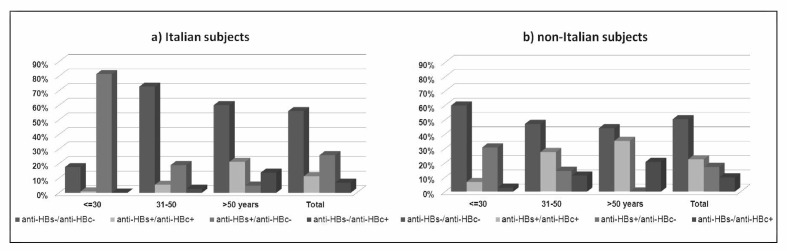
Table I and Figure 2 present the differences in antibody patterns between Italian and non-Italian subjects stratified by age. The difference in the prevalence between Italian and non-Italian subjects was statistically significant for all these patterns in subjects ≤30 years old, while it was not in older age classes.
Table I.
HBV serological patterns by age in Italian and non-Italian HBsAg-negative subjects (2007–2008).
| Age classes (years) | N | Anti-HBs+/anti-HBc+ (95% CI) | Anti-HBs+/anti-HBc– (95% CI) | Anti-HBs/anti-HBc+ (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| Italians | Non-Italians | Italians | Non-Italians | p | Italians | Non-Italians | p | Italians | Non-Italians | p | |
| ≤30 | 950 | 75 | 9 (0.9%) (0.30–1.50) | 5 (6.7%) (1.04–12.36) | <0.01 | 773 (81.4%) (78.93–83.87) | 23 (30.7) (20.26–41.14) | <0.01 | 1 (0.1%) (0.00–0.30) | 2 (2.7%) (0.00–6.37) | <0.05 |
| 31–50 | 1,690 | 153 | 93 (5.5%) (4.41–6.59) | 42 (27.5%) (20.42–34.57) | <0.01 | 321 (19.0%) (17.13–20.87) | 22 (14.4%) (8.84–19.96) | NS | 45 (2.7%) (1.93–3.47) | 17 (11.1%) (6.12–16.08) | <0.01 |
| >50 | 1,971 | 34 | 417 (21.2%) (19.39–23.00) | 12 (35.3%) (19.24–51.36) | NS | 96 (4.9%) (3.95–5.85) | 0 (0%) (0.00–0.00) | NS | 272 (13.8%) (12.28–15.32) | 7 (20.6%) (7.01–34.19) | NS |
| Total | 4,611 | 262 | 519 (11.3%) (10.39–12.21) | 59 (22.5%) (17.44–27.56) | <0.01 | 1,190 (25.8%) (24.54–27.06) | 45 (17.2%) (12.63–21.77) | <0.01 | 318 (6.9%) (6.17–7.63) | 26 (9.9%) (6.28–13.52) | NS |
NS=not significant
Figure 2.
Prevalence of HBV serological patterns by age in Italian and non-Italian HBsAg negative subjects (2007–2008).
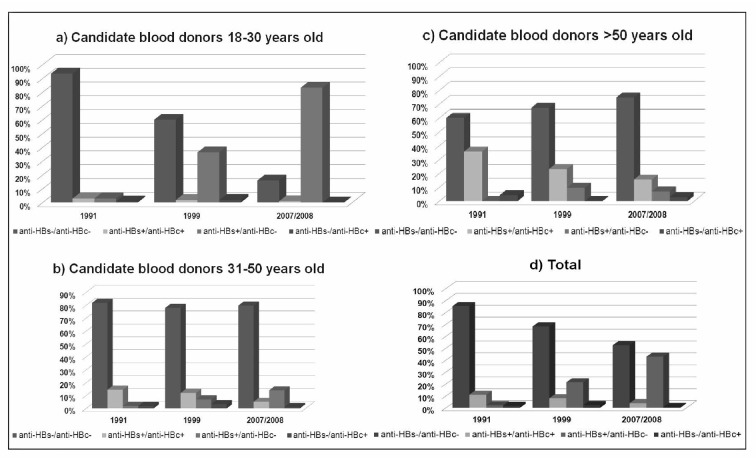
The data relating to the candidate blood donors are shown in Table II and are compared to data from 1991 and 1999, stratified by age, in Figure 3. The prevalence of the anti-HBs positive/anti-HBc negative pattern increased from 2.2% (95% CI: 0.92–3.48) in 1991 to 21.4% (95% CI: 18.04–76) in 1999 and 42.9% (95% CI: 40.27–45.53) in 2007/2008. Conversely, the prevalence of the anti-HBs positive/anti-HBc positive pattern decreased from 11.0% (95% CI: 8.28–13.72) in 1991 to 8.1% (95% CI: 5.86–10.34) in 1999 and 3.9% (95% CI: 2.87–4.93) in 2007/2008. The prevalence of the anti-HBs negative/anti-HBc positive pattern varied from 1.6% (95% CI: 0.51–2.69) in 1991 to 2.3 % (95% CI: 1.07–3.53) in 1999 and 0.7% (95% CI: 0.26–1.14) in 2007/2008. This trend was present in all age classes (Figure 3). In 2007/2008 the difference in the prevalence of the anti-HBs positive/anti-HBc positive and anti-HBs positive/anti-HBc negative patterns in Italian and non-Italian candidate blood donors was statistically significant (p<0.01).
Table II.
HBV serological patterns in Italian and non-Italian HBsAg-negative candidate blood donors (2007–2008).
| Markers | Origin | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Italian | Non-Italian | p | Total | ||||
|
| |||||||
| N (%) | 95% CI | N (%) | 95% CI | N (%) | 95% CI | ||
| Anti-HBs neg/anti-HBc neg | 698 (52.3) | 49.62–54.98 | 14 (58.3) | 38.57–78.03 | NS | 712 (52.4) | 49.74–55.06 |
| Anti-HBs pos/anti-HBc pos | 46 (3.4) | 2.43–4.37 | 7 (29.2) | 11.01–47.39 | <0.01 | 53 (3.9) | 2.87–4.93 |
| Anti-HBs pos/anti-HBc neg | 581 (43.6) | 40.94–46.26 | 2 (8.3) | 0.00–19.34 | <0.01 | 583 (42.9) | 40.27–45.53 |
| Anti-HBs neg/anti-HBc pos | 9 (0.7) | 0.25–1.15 | 1 (4.2) | 0.00–12.23 | NS | 10 (0.7) | 0.26–1.14 |
| Total | 1,334 | 24 | 1,358 | ||||
NS=not significant
Figure 3.
Prevalence of HBV markers in candidate blood donors by age in 1991, 1999 and 2007/2008.
Discussion
The epidemiology of HBV in Italy has changed considerably over the last 20 years. Improved socio-economic conditions and hygiene and the introduction of the anti-HBV vaccination campaign led Italy to become a low HBV endemic country, with a prevalence of HBsAg carriers in the population of less than 2%20.
On the other hand, increasing immigration from highly endemic countries has led to changes in HBV epidemiology. In addition to the higher prevalence of HBsAg among these subjects, even those who are negative display the highest prevalence of the markers of past infection31,32. The global prevalence of anti-HBc in our study was 19.1%. This figure was partly due to the presence of non-Italians, among whom the prevalence was almost twice that among Italian subjects, thus reflecting their origin from countries in which HBV is still endemic.
There was no between-group difference in the prevalence of anti-HBs but, in the case of the simultaneous presence of anti-HBs and anti-HBc, the anti-HBs was more related to past infections among the non-Italians and to vaccinations among the Italians. This is best appreciated by observing the pattern of markers according to age. Among the Italians, the frequency of markers of past infection was low up to 30 years, but it was higher in the older age groups. This is due to a cohort effect, as the majority of the infections were acquired in the past. In contrast, the prevalence of anti-HBs alone (as a marker of a response to vaccination) was higher in the younger age groups mainly targeted by the vaccination campaign. In comparison with Italians, a smaller percentage of non-Italians had anti-HBs due to vaccination in all the age groups, whereas the prevalence of markers of past infection was always higher.
As far as the isolated anti-HBc pattern is concerned (anti-HBs negative/anti-HBc positive), its prevalence in the Italian subjects increases with age, and is as high as about 14% in those aged more than 50 years (probably due to a loss of anti-HBs in subjects infected long before). It is stated in the literature that an isolated anti-HBc pattern should be carefully evaluated because of false positive results of anti-HBc reactivity tests (particularly with enzyme immunoassays)33,34. We used a screening enzyme immunoassay with a declared specificity of 99.75% and, in case of an isolated anti-HBc pattern, the positive results were confirmed by an enzyme linked fluorescent assay with a declared specificity of 99.94%, so our results can be considered sufficiently reliable.
It is possible that some of our cases with isolated anti-HBc may have had occult infection as there are published data on blood donors indicating an association between an isolated anti-HBc pattern and the concomitant presence of HBV DNA and infectious blood units35,36. Moreover, HBV DNA has been found in the presence of anti-HBs as well as anti-HBc in the case of occult infections in blood donors37,38. However, we could not investigate this any further because of the retrospective nature of our study. Another limitation of our work is the very small number of subjects younger than 11 years (only 31 subjects, data not shown) and the lack of data about the percentage of individuals with anti-HBs and anti-HBc or isolated anti-HBc who had been vaccinated.
In candidate blood donors the prevalence of markers of past infection and of vaccination are respectively lower and higher than in in-patients and out-patients, especially in the 31 to 50-year old age class, in which the major part of candidate blood donors can be found. However this group represents an healthy population, while (in- and out-)patients constitute a more varied population.
The comparison of data concerning Italian candidate blood donors with those obtained in a previous study on similar subjects in 1991 and 1999 clearly shows the decrease in the markers of past infection over time in all age groups, and a corresponding increase in the number of anti-HBs-positive subjects due to vaccination. The prevalence of HBsAg among the subjects who represent the generally healthy population in our area was 0.6% (currently in the course of publication). Non-Italians blood donors accounted for 1.8% of the total and, once again, they had a higher prevalence of markers of past infection than the Italians. The number of non-Italian blood donors is still very low in our area. Blood donation in Italy is not paid and does not, therefore, attract people seeking a means of increasing their income as in some other countries, but requires a certain level of cultural and economic integration. Probably only in the coming years will there be an increase of blood donors from recent waves of immigration. Unfortunately we have no data for anti-HBs and anti-HBc prevalence in the regular donors of 2007/2008, unlike those in 1991 and 199930, since these tests were recently performed only on candidate blood donors.
In conclusion, as expected after the introduction of vaccination, the prevalence of the markers of past HBV infection in the Italian population of our area has decreased, but the increasing number of largely unvaccinated immigrants from highly endemic countries who show a high prevalence of the serological markers of active and past infections is redefining the epidemiology of HBV infection. This could be particularly important in transfusion medicine. The guidelines of the Italian Society of Transfusion and Immunohaematology (SIMTI) do not currently recommend an anti-HBc test to determine the eligibility of donors or blood components because its addition to testing for HBsAg and HBV-DNA seems to give no advantage to transfusion safety in the current epidemiological situation22. The arrival of larger numbers of people from countries in which HBV is highly endemic, in association with a residual risk of transfusional HBV transmission which is still higher than that for other viruses (hepatitis C virus and human immunodeficiency virus), may lead in the near future to a revision of the prevention strategies for reducing transfusion risk.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Romanò L, Paladini S, Van Damme P, Zanetti AR. The worldwide impact of vaccination on the control and protection of viral hepatitis B. Dig Liv Dis. 2011;43(Suppl. 1):S2–7. doi: 10.1016/S1590-8658(10)60685-8. [DOI] [PubMed] [Google Scholar]
- 2.Merrill RM, Hunter BD. Seroprevalence of markers for hepatitis B viral infection. Int J Infect Dis. 2011;15:e78–121. doi: 10.1016/j.ijid.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 3.Torbenson M, Thomas DL. Occult hepatitis B. Lancet Infect Dis. 2002;2:479–86. doi: 10.1016/s1473-3099(02)00345-6. [DOI] [PubMed] [Google Scholar]
- 4.Almeida Neto C, Strauss E, Sabino EC, et al. Significance of isolated hepatitis B core antibody in blood donors from Sao Paulo. Rev Inst Med Trop Sao Paulo. 2001;43:203–8. doi: 10.1590/s0036-46652001000400005. [DOI] [PubMed] [Google Scholar]
- 5.Douglas DD, Taswell HF, Rakela J, Rabe D. Absence of hepatitis B virus DNA detected by polymerase chain reaction in blood donors who are hepatitis B surface antigen negative and antibody to hepatitis B core antigen positive from a United States population with a low prevalence of hepatitis B serologic markers. Transfusion. 1993;33:212–6. doi: 10.1046/j.1537-2995.1993.33393174446.x. [DOI] [PubMed] [Google Scholar]
- 6.Kitchen AD, Harrison TJ, Meacock TJ, et al. Incidence and significance of hepatitis B core antibody in a healthy blood donor population. J Med Virol. 1988;25:69–75. doi: 10.1002/jmv.1890250110. [DOI] [PubMed] [Google Scholar]
- 7.Joller-Jemelka HI, Wicki AN, Grob PJ. Detection of HBs antigen in “anti-HBc alone” positive sera. J Hepatol. 1994;21:269–72. doi: 10.1016/s0168-8278(05)80407-6. [DOI] [PubMed] [Google Scholar]
- 8.Hennig H, Puchta I, Luhm J, et al. Frequency and load of hepatitis B virus DNA in first-time blood donors with antibodies to hepatitis B core antigen. Blood. 2002;100:2637–41. doi: 10.1182/blood-2002-03-0798. [DOI] [PubMed] [Google Scholar]
- 9.Lok AS, Lai CL, Wu PC. Prevalence of isolated antibody to hepatitis B core antigen in an area endemic for hepatitis B virus infection: implications in hepatitis B vaccination programs. Hepatology. 1988;8:766–70. doi: 10.1002/hep.1840080411. [DOI] [PubMed] [Google Scholar]
- 10.Bernvil SS, Andrews V, Kuhns MC, McNamara AL. Hepatitis B core antigen antibody as an indicator of a low grade carrier state for hepatitis B virus in a Saudi Arabian blood donor population. Transfus Sci. 1997;18:49–53. doi: 10.1016/s0955-3886(96)00076-8. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization Hepatitis B Fact Sheet No. 204 (Revised October 2008). ( http://who.int/mediacentre/factsheets/fs204/en).
- 12.Ng KP, Saw TL, Baki A, et al. Impact of the Expanded Program of Immunization against hepatitis B infection in school children in Malaysia. Med Microbiol Immunol. 2005;194:163–8. doi: 10.1007/s00430-004-0231-4. [DOI] [PubMed] [Google Scholar]
- 13.Ni YH, Huang LM, Chang MH, et al. Two decades of universal hepatitis B vaccination in Taiwan: impact and implication for future strategies. Gastroenterology. 2007;132:1287–93. doi: 10.1053/j.gastro.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 14.Piazza M, Da Villa G, Picciotto L, et al. Mass vaccination against hepatitis B in infants in Italy. Lancet. 1988;2:1132. doi: 10.1016/s0140-6736(88)90540-5. [DOI] [PubMed] [Google Scholar]
- 15.Zanetti AR, Tanzi E, Romanò L, Grappasonni I. Vaccination against hepatitis B: the Italian strategy. Vaccine. 1993;11:521–4. doi: 10.1016/0264-410x(93)90222-j. [DOI] [PubMed] [Google Scholar]
- 16.Stroffolini T, Guadagnino V, Chionne P, et al. A population based survey of hepatitis B virus infection in a southern Italian town. Ital J Gastroenterol Hepatol. 1997;29:415–8. [PubMed] [Google Scholar]
- 17.Romanò L, Paladini S, Tagliacarne C, et al. The changing face of the epidemiology of type A, B, and D viral hepatitis in Italy, following the implementation of vaccination. Vaccine. 2009;27:3439–42. doi: 10.1016/j.vaccine.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 18.Stroffolini T, Mele A, Tosti ME, et al. The impact of hepatitis B mass immunisation campaign on the incidence and risk factors of acute hepatitis B in Italy. J Hepatol. 2000;33:980–5. doi: 10.1016/s0168-8278(00)80132-4. [DOI] [PubMed] [Google Scholar]
- 19.Mele A, Tosti ME, Mariano A, et al. Acute hepatitis B 14 years after implementation of universal vaccination in Italy: areas of improvement and emerging challenges. Clin Infect Dis. 2008;46:868–75. doi: 10.1086/528687. [DOI] [PubMed] [Google Scholar]
- 20.Sagnelli E, Stroffolini T, Mele A, et al. Italian Hospitals’ Collaborating Group Chronic hepatitis B in Italy: new features of an old disease - approaching the universal prevalence of hepatitis B and antigen-negative cases and the eradication of hepatitis D infection. Clin Infect Dis. 2008;46:110–3. doi: 10.1086/524074. [DOI] [PubMed] [Google Scholar]
- 21.Giusti G, Gaeta GB, Russo M, Bedarida G. HBsAg carriers among blood donors in Italy-a multicentre study in 107 blood banks. Infection. 1989;17:237–9. doi: 10.1007/BF01639527. [DOI] [PubMed] [Google Scholar]
- 22.Velati C, Fomiatti L, Baruffi L, et al. Criteria for hepatitis B virus screening and validation of blood components in Italy: the position of the SIMTI HBV working group. Blood Transfus. 2011;9:455–61. doi: 10.2450/2011.0014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stroffolini T. The changing pattern of hepatitis B virus infection over the past three decades in Italy. Dig Liver Dis. 2005;37:622–7. doi: 10.1016/j.dld.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Fabris P, Baldo V, Baldovin T, et al. Changing epidemiology of HCV and HBV infections in Northern Italy: a survey in the general population. J Clin Gastroenterol. 2008;42:527–32. doi: 10.1097/MCG.0b013e318030e3ab. [DOI] [PubMed] [Google Scholar]
- 25.Da Villa G, Romanò L, Sepe A, et al. Impact of hepatitis B vaccination in a highly endemic area of south Italy and long-term duration of anti-HBs antibody in two cohorts of vaccinated individuals. Vaccine. 2007;25:3133–6. doi: 10.1016/j.vaccine.2007.01.044. [DOI] [PubMed] [Google Scholar]
- 26.D’Amelio R, Matricardi PM, Biselli R, et al. Changing epidemiology of hepatitis B in Italy: public health implications. Am J Epidemiol. 1992;135:1012–8. doi: 10.1093/oxfordjournals.aje.a116395. [DOI] [PubMed] [Google Scholar]
- 27.Salvaneschi L, Poma R, Pagani A. I problemi virali delle trasfusioni. In: Bombardieri E, Cavallo G, Landolfo S, editors. Argomenti di Laboratorio per la Virologia. Brescia: Edizioni CLAS International; 1990. p. 179. [Google Scholar]
- 28.Manzini P, Girotto M, Borsotti R, et al. Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica. 2007;92:1664–70. doi: 10.3324/haematol.11224. [DOI] [PubMed] [Google Scholar]
- 29.Caritas migrantes . Immigrazione Dossier Statistico 2008 XVIII rapporto. Roma: Edizioni Idos; 2008. [Google Scholar]
- 30.De Paschale, Biagiotti S, Chianese R, et al. Variazione nel tempo della prevalenza dei marcatori dell’epatite B in donatori di sangue. La Trasfusione del Sangue. 2001;46:335–40. [Google Scholar]
- 31.Tafuri S, Prato R, Martinelli D, et al. Prevalence of hepatitis B, C, HIV and syphilis markers among refugees in Bari, Italy. BMC Infect Dis. 2010;10:213. doi: 10.1186/1471-2334-10-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Majori S, Balbo V, Tommasi I, et al. Hepatitis A, B, and C infection in a community of sub-Saharan immigrants living in Verona (Italy) J Travel Med. 2008;15:323–7. doi: 10.1111/j.1708-8305.2008.00230.x. [DOI] [PubMed] [Google Scholar]
- 33.McMahon BJ, Parkinson AJ, Helminiak C, et al. Response to hepatitis vaccine of persons positive for antibody to hepatitis B core antigen. Gastroenterology. 1992;103:590–4. doi: 10.1016/0016-5085(92)90851-o. [DOI] [PubMed] [Google Scholar]
- 34.Parkinson AJ, McMahon BJ, Hall D, et al. Comparison of enzyme immunoassay with radioimmunoassay for the detection of antibody to hepatitis B core antigen as the only marker of hepatitis B infection in a population with a high prevalence of hepatitis B. J Med Virol. 1990;30:253–7. doi: 10.1002/jmv.1890300405. [DOI] [PubMed] [Google Scholar]
- 35.Zanetti AR, Romanò L, Zappa A, Velati C. Changing patterns of hepatitis B infection in Italy and NAT testing for improving the safety of blood supply. J Clin Virol. 2006;36(Suppl. 1):S51–5. doi: 10.1016/s1386-6532(06)80009-0. [DOI] [PubMed] [Google Scholar]
- 36.Velati C, Romanò L, Fomiatti L, et al. For the Italian Group for the Study of Tranfusion-Transmissible Diseases. HBV DNA detection in HBsAg neg/anti-core positive Italian first time blood donors. Vox Sang. 2006;91(Suppl. 3):66. [Google Scholar]
- 37.Velati C, Romanò L, Fomiatti L, et al. SIMTI Research Group Impact of nucleic acid testing for hepatitis B virus, hepatitis C virus, and human immunodeficiency virus on the safety of blood supply in Italy: a 6-year survey. Transfusion. 2008;48:2205–13. doi: 10.1111/j.1537-2995.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 38.Raimondo G, Allain JP, Brunetto MR, et al. Statements from the Taormina expert meeting on occult hepatitis B virus infection. J Hepatol. 2008;49:652–7. doi: 10.1016/j.jhep.2008.07.014. [DOI] [PubMed] [Google Scholar]