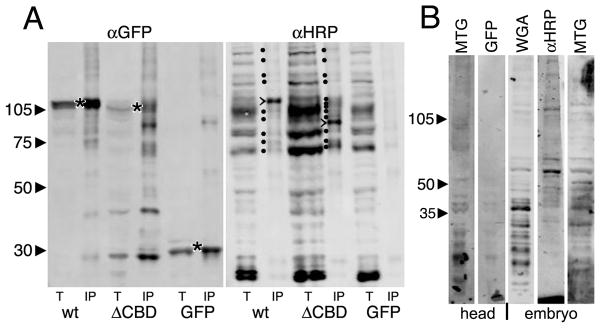
Fig 8. Both wildtype and ΔCBD MTG bind HRP-epitope glycoproteins.
(A) Representative Western Blots of total lysate (t) or immunoprecipitate (IP) from wandering 3rd instar salivary gland lysates. Wildtype (wt) MTG::GFP, ΔCBD MTG::GFP and GFP alone were IP isolated using anti-GFP (left), and probed with α-HRP (right). Left (αGFP): all three proteins are pulled down (*). The ΔCBD protein is slightly smaller than wt protein, as expected. Right: (α-HRP): The total lysates show multiple bands, with a few bands (marked with dots) pulled down by wildtype MTG::GFP. One major band migrates at ~115 kDa. ΔCBD pulls down more HRP epitope proteins, with a major band at ~94 kDa (major bands are marked with >). (B) Using MTG::GFP as a probe in a Far-Western Blot, wandering 3rd instar salivary gland lysates from animals expressing MTG::GFP, or GFP alone, were applied to membranes blotted with proteins of head tissue (2 lanes, left) or embryos (3 lanes, right). GFP alone shows no consistent binding to any protein bands, whereas MTG::GFP consistently binds many protein bands. αHRP antibody and WGA lectin recognize similar glycoproteins.